John E. Rush, DVM, MS, Diplomate ACVIM (Cardiology), Diplomate ACVECC

Chronic valve disease (CVD) is the most frequent cause of congestive heart failure in dogs. This condition has also been termed chronic degenerative valvular disease, myxomatous atrioventricular valvular degeneration, chronic valvular fibrosis and endocardiosis. The incidence is reported as being between 11% (clinical determination) and 42% (necropsy determination) depending on the method of examination. The incidence of CVD is increased further, to above 60%, in aged dogs. The mitral valve alone is affected in 60% of dogs with CVD, with the mitral and tricuspid valves both affected in 30% of cases and the tricuspid valve alone in 10% of dogs. The aortic and pulmonic valves may be affected but clinically important disease is uncommon. Signs of mitral valve disease and left-sided cardiac dysfunction predominate in most cases.
SIGNALMENT
The disease is most common in small to medium sized breeds of dogs. Cavalier King Charles Spaniels and Dachshunds are over-represented. CVD also occurs in large breed dogs (i.e., German Shepherd dog, Doberman pinscher), but dilated cardiomyopathy is much more common in these breeds than CVD. The incidence of CVD is increased in male dogs relative to females (1.5 to 1.0). This is a slowly progressive disease in which lesions may begin in the first half of life (e.g., 2 to 3 years), but clinical disease is unlikely before middle age. In the early stages, the only clinical sign may be a cardiac murmur detected on routine examination. Cardiac decompensation and congestive heart failure (CHF) typically occurs in later life (e.g., 6 to 10 years of age or older).
ETIOLOGY
The etiology is unknown. A genetic tendency to develop the disease has been proved in the Cavalier King Charles Spaniel.
PATHOLOGY
Valve leaflets may be grossly thickened and shortened with curled, nodular margins. There is fibrosis of the valves with an accumulation of acid mucopolysaccharide ground substance. Valvular hemorrhage and calcification may be seen. Chordae tendineae are thickened and may rupture. The disease is primarily a non-inflammatory, myxomatous degeneration of the A-V valve that is commonly referred to as endocardiosis. Many dogs with chronic mitral regurgitation have histologic evidence of small foci of myocardial fibrosis and necrosis, as well as intramural coronary arteriosclerosis (referred to as microscopic intramural myocardial infarction or MIMI). The left ventricle is dilated and hypertrophied if there has been significant volume overload. The left atrium is dilated and, in severe cases, the endocardium may split and rupture creating cardiac tamponade. The onset of congestive heart failure is associated with chronic passive congestion of the lungs (left-sided CHF) or abdominal viscera (right-sided CHF).
PATHOPHYSIOLOGY
Valvular regurgitation results in a volume overload of the associated ventricle. Classic compensatory mechanisms (sympathetic nervous system, renin-angiotensin system, etc.) are activated to restore blood pressure and tissue perfusion towards normal. In compensated mitral regurgitation, cardiac remodeling occurs with ventricular dilatation and left ventricular (LV) wall hypertrophy. The left atrium (LA) is dilated and, as long as the atrium remains compliant enough to accept the regurgitated blood, CHF does not develop. Eventually LA pressure rises and pulmonary edema develops. In the early to middle stages of the disease the LV wall thickness in increased and LV end- diastolic volume is greater than normal, yet the LV contracts down to a normal end-systolic volume (resulting in an increase in fractional shortening). Chronic mitral regurgitation eventually leads to myocardial dysfunction and myocardial failure, which can be manifest by increasing LV end systolic volume or decreasing fractional shortening. Chronic left heart failure leads to pulmonary hypertension, posing added strain to the right heart and contributing to signs of right-sided CHF in dogs with concurrent CVD of the tricuspid valve. There is reasonably good experimental evidence to indicate that dogs with significant mitral regurgitation have more compromise to myocardial systolic function than is evident from the "apparently" good systolic function seen on echocardiography. The leaking mitral valve allows a low pressure "pop-off" which permits a large volume of blood to be pumped into a low pressure chamber, allowing for a high fractional shortening on the echocardiogram despite significant myocardial dysfunction.
CLINICAL SYNDROMES
A wide range of clinical presentations are possible, depending on the degree and duration of valvular dysfunction. Many older dogs presented for routine examination or for other medical problems are identified to have the typical murmur of mitral (MR) or tricuspid (TR) regurgitation. Baseline testing (e.g., radiographs, echocardiography) is often helpful for comparison at subsequent examinations. In many dogs CHF is the cause for initial evaluation with cough, nocturnal dyspnea, altered sleep habits, abdominal distention or exercise intolerance as the presenting complaints. Congestive heart failure may be manifest as pulmonary edema, pleural effusion, or ascites. Acute, fulminant CHF result from rupture of a chorda tendineae or exacerbation of chronic CHF following a stressful or decompensating event (i.e., anesthesia/surgery, high sodium treats/diet, onset of rapid tachyarrhythmia, etc.). In dogs with advanced CVD and mitral regurgitation the LA may become large enough to put pressure on the large airways creating cough due to large airway compression. Tracheal and large airway collapse, concurrent bronchitis, and other respiratory diseases are common in small breed dogs and can contribute to cough, leading to confusion as to the most important cause of cough. Syncope can occur due to significant arrhythmias, cardiac output, or following a coughing spell (e.g., tussive syncope). In the author's experience, syncope occurring at the time of the initial presentation for heart failure usually resolves following resolution of pulmonary edema. Finally, endocardial splitting with left atrial rupture can result in acute collapse due to cardiac tamponade. In these cases, signs of reduced forward blood flow (weakness, hypotension) predominate; the lungs can be free of pulmonary edema.
HISTORY
Cough is the most common presenting complaint in dogs with clinical signs resulting from CVD. The cough is often described as a deep, resonant cough or a hacking cough. The cough may occur after exercise or excitement, or it may be more prominent at night. Additional historical complaints can include dyspnea, orthopnea, respiratory distress, reduced exercise tolerance, weight loss (cardiac cachexia), progressive abdominal distention due to right-sided CHF, and syncope.
PHYSICAL EXAMINATION
The classic auscultation finding is a systolic cardiac murmur in the typical mitral or tricuspid valve location. An extra systolic sound known as a mid-systolic "click" has been associated with early CVD and mitral valve prolapse. A mid-systolic click is likely the earliest auscultatory finding for CVD, but it can be easily missed or mistaken for a cardiac gallop. There is a tendency for the intensity of the murmur to be roughly correlated with the severity of cardiac dysfunction. The initial murmur of mitral regurgitation may not persist for the entire duration of systole and is localized to the 5th intercostal space at left apex. With increasing disease severity, the murmur becomes holosystolic and more intense, radiating cranially, dorsally, and to the right. When sufficiently intense, the murmur can radiate to the right mid-thorax, making it difficult to discern the presence of a second murmur due to tricuspid regurgitation. The murmur of tricuspid regurgitation is located on the right hemithorax, roughly midway between the cardiac apex and base. An S3 gallop is commonly heard in dogs with CHF or myocardial failure.
Dogs with CHF may have tachypnea, hyperpnea, dyspnea, or orthopnea, and anxiety with a reluctance to lie down. A cough may be elicited by gentle tracheal palpation or may occur unprovoked. Paroxysms of cough may end in swallowing or the production of a white foam; in advanced cases, a blood tinged froth is produced. Mucous membranes are pink in early stages, but may progress to a muddy to somewhat cyanotic color with decompensation and severe left-sided CHF. Pulmonary auscultation may also reveal increased respiratory sounds which progress to crackles with the onset of alveolar edema. The latter may be particularly prominent over the hilar or caudal lung fields on inspiration. Hepatomegaly and ascites may be evident in dogs with right-sided CHF from advanced disease, and in these cases the jugular veins are typically distended and/or exhibit a prominent systolic "V" wave due to the ejection of blood into the RA. The femoral pulses are often easily palpated and prominent, but may exhibit a jerky "collapsing" character due to the abbreviation of LV ejection. Arterial pulses often become weak in animals with advanced CHF or myocardial failure. There may also be irregularities in pulse rate and strength resulting from cardiac arrhythmia. In animals with CHF, the heart rate is usually elevated or in the upper normal range and sinus arrhythmia is typically absent. The ventricular apex beat is hyperdynamic and is progressively shifted caudally from the fifth intercostal space with increasing disease severity. When present, a precordial thrill is also palpated in this region.
THORACIC RADIOGRAPHY
The earliest finding on thoracic radiographs is left atrial enlargement (Figure 1A and 1B). On the lateral projection, the left atrium is visualized at the caudodorsal aspect of the cardiac silhouette. The left atrium enlarges progressively with advancing disease. Left atrial and left ventricular enlargement result in elevation of the trachea and carina, with a decrease in the angle made between the trachea and the thoracic spine. The left mainstem bronchus may become elevated (compressed) in cases of marked left atrial enlargement. There is straightening of the caudal cardiac border and loss of the caudal cardiac waist. Pulmonary venous dilation occurs and is often evaluated in the cranial lung fields in this view. Early pulmonary edema is seen as a diffuse increase in interstitial density in the hilar or caudal lung fields, progressing to fluffy densities and air bronchograms with the onset of alveolar edema. With tricuspid regurgitation and right- sided or biventricular CHF, the cranial aspect of the trachea may be elevated and the caudal vena cava will increase in size. Hepatomegaly and splenomegaly may be present. On the DV or VD radiographic projection, the earliest findings in CVD is enlargement of the left atrial appendage, located at the 2 to 3 o'clock position (using the clockface analogy). Left ventricular enlargement leads to rounding of the LV apex, which may be displaced to the left. With progressive enlargement of the left atrium there is an increase in the angle formed by the right and left main stem bronchi (resulting in the bow-legged cowboy sign). Right atrial and ventricular enlargement leads to rounding of the cardiac silhouette from the 6:00 to 11:00 position. Pulmonary veins (and arteries in severe cases) are distended in the caudal lung fields and the previously noted pulmonary parenchymal changes are evident when left-sided CHF develops.
ELECTROCARDIOGRAPHY
ECG findings include evidence of left ventricular hypertrophy, left atrial enlargement (P mitrale; P > 0.04 sec), and infrequently P pulmonale (P > 0.4 mv). ST segment slurring is seen in some dogs with left ventricular hypertrophy, and ST depression may result from hypoxemia. Sinus rhythm or sinus tachycardia with a normal mean electrical axis are typical. Supraventricular arrhythmias occur commonly. Atrial fibrillation develops in some dogs with marked atrial enlargement and ventricular arrhythmias are uncommon in dogs with compensated disease.
ECHOCARDIOGRAPHY
Valvular thickening may be readily appreciated in most dogs with CVD. This valvular thickening and irregularity is most easily appreciated in the anterior mitral leaflet. With severe disease or with rupture of the chordae tendinae the valve motion takes on a vigorous, chaotic quality. The mitral valve leaflet can be noted to prolapse into the LA during systole. Left ventricular dilation is present to a variable degree depending upon disease severity. Progressive left atrial dilation occurs in most cases. Fractional shortening is increased with increasing regurgitant fraction, however fractional shortening can return toward "normal" or become diminished with disease progression and myocardial failure. End-systolic dimension or end-systolic volume index may be a better indication of myocardial function as these dimensions tend to increase with impending myocardial failure. As a crude indication of disease severity, the location and extent of the regurgitant jet can be estimated using color flow (CFD) Doppler echocardiography. While the degree of mitral or tricuspid regurgitation on color-flow Doppler may provide some qualitative information, the degree of disease progression is probably better characterized by left atrial size and measures of left ventricular function.
CHRONIC VALVULAR DISEASE IN CAVALIER KING CHARLES SPANIELS
Cavalier King Charles Spaniels are known to get CVD early in life. Absence of a cardiac murmur before 5 years of age in male dogs and before 6 years of age in female dogs has been proposed as a criterion for recommendations of populations for breeding. Most dogs will live three to four years after development of a cardiac murmur. Necropsy results of a small percentage of those CKCS examined revealed that mitral valve prolapse and ruptured chordae tendineae are major findings with CVD in this breed. More specifically, those tendineae associated with the medial aspect of the anterior mitral valve leaflet are most commonly ruptured, often in the absence of significant thickening due to endocardiosis. A study of enalapril use in this breed to prevent progression of the disease or delay the onset of CHF failed to identify a significant clinical benefit in asymptomatic dogs.
TREATMENT OF DOGS WITH CVD
The optimal treatment of dogs with CVD remains a topic of some discussion and debate. A variety of drugs and diets have been develop to manage the disease, however little research has been done to identify the cause of the valvular degeneration. If the cause of endocardiosis can be identified and treated then the disease could be slowed or reversed. Until that time, management of congestive heart failure (CHF) remains the cornerstone of therapy for CVD. Clinical trials of human heart failure patients have documented that use of certain therapies (e.g., Angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, spironolactone) leads to improved survival. The ACE inhibitors have been studied in dogs with CVD, however there is little information available regarding use of beta-blockers or spironolactone.
Angiotensin-converting enzyme (ACE) inhibitors are commonly used in the management of CVD. One published study and data from another recently presented study failed to identify a clear benefit from early use of ACE inhibitors in dogs with CVD that have not yet developed clinical signs. However, once clinical evidence of heart failure is present, use of ACE inhibitors is appropriate and is also typically safe in the absence of significant pre-existing renal disease or excessive concurrent diuretic use. In general, ACE inhibitors should be used in all dogs with CVD that have developed CHF, assuming drug therapy is well tolerated.
Diuretic therapy is indicated in any dog with evidence of congestion (e.g., pulmonary edema, pleural effusion, or ascites of cardiogenic origin). It can be quite difficult to define the exact dose of diuretic required by any individual dog. The dose required to clear significant edema accumulations and cause the animal to be minimally symptomatic (the desired dose) is often close to a dose that might result in electrolyte disturbance, dehydration and the development of pre-renal azotemia. The combined use of ACE inhibitors and diuretics compromises one of the kidneys' normal compensatory mechanisms (vasoconstriction of the efferent arteriole) and can lead to elevation of BUN and creatinine if an excessive diuretic dose is initiated. Diuretic dose should be reduced in dogs that are weak, lethargic, anorexic, or depressed, especially if congestive signs are well controlled. In most instances of mild CHF in dogs a furosemide dose of 2 mg/kg q 12-24 hours will control edema formation. When the furosemide dose of 2.2 mg/kg twice a day is exceeded during chronic therapy, diuretic resistance has been likely occurred and the author recommends addition of diuretics with differing mechanisms of action. Spironolactone has been documented to result in improved survival in a human CHF study, however information on the optimal dose and time of initiation of this drug is veterinary medicine is largely anecdotal. In cases of refractory CHF, a combination of hydrochlorothiazide with spironolactone is recommended. Serial electrolyte monitoring is advisable as spironolactone use can lead to mild hyperkalemia, and hydrochlorothiazide can result in hyponatremia and hypochloremia. Digitalis glycosides are also useful to control advanced CHF. The author most commonly uses digoxin as an add-on therapy to ACE inhibitors and diuretics for refractory CHF or in dogs with significant supraventricular arrhythmias.
Beta blockade has gained favor recently as a therapeutic modality for treatment of CHF. Several studies on the use of beta-blockers have documented benefits that accrue from chronic treatment, although these effects are often not seen for several months. Reported benefits include up regulation of previously down regulated beta-receptors, improved cardiac performance (improved stroke volume), and improved survival. These clinical benefits appear to have sound theoretical basis, and are currently being evaluated in dogs with naturally occurring CHF. It is true that beta-blockers are negative inotropes, and this may be the most demonstrable effect when these drugs are used in animals with active CHF. The negative inotropic and negative chronotropic effects of the drugs will likely contribute to worsening of CHF in animals with active failure or those that are on the edge of compensation. Beta-blockers are best employed in animals that are minimally symptomatic with early/mild heart failure, or in animals in later stages of CHF that are already well controlled on a stable cardiac drug regimen.
Sodium restricted diets and exercise restriction have long been a useful additions to the management of CHF in dogs. The optimal time to initiate a cardiac diet and the ideal sodium level relative to the severity of clinical disease are areas for debate and further research. A diet designed for early use in dogs with CVD has been recently developed (Waltham Early Cardiac Support Diet) and studies of this diet are ongoing. Exercise restriction with severe congestive heart failure is essential, however limited exercise in dogs with stable chronic heart failure is often well tolerated. Repetitive or strenuous activities like ball chasing and running should be restricted.
Surgical procedures are being developed to repair or replace the mitral valve in dogs with CVD. Cardiopulmonary bypass is required for these surgeries, and successful cardiopulmonary bypass requires a dedicated team of surgeons, perfusionist, anesthesiologist, cardiologist, intensive care specialist, and strong veterinary technician support. The cost associated with surgery can be prohibitive, however cardiopulmonary bypass and mitral valve surgery have become routine in the human field. Once these techniques are mastered and refined for veterinary medicine it seems probable that surgery will become the preferred therapy for those that can afford the procedure.
RE-EVALUATION AND FOLLOW-UP
The author routinely recommends re-evaluation with a physical examination and chemistry profile to check renal function and electrolytes 7 to 10 days after initiation or alteration of cardiac medications. Serum digoxin levels should be obtained if this drug is used, ideally 8 hours post-pill. Additional tests that might be appropriate at the time of the initial recheck examination include packed cell volume and total proteins to help assess hydration, blood pressure, follow-up thoracic radiographs in dogs with CHF, and follow-up electrocardiography in animals with arrhythmia. The next visit should be scheduled for 2 to 3 months and at that time a physical examination with chemistry profile should be performed. Approximately, 6 months after initial diagnosis the author recommends a follow up examination with echocardiography to search for changes in the appearance of the heart or other alterations which might dictate a need for change in therapy.
Figure 1 A and B
Lateral (A) and dorsoventral (B) thoracic radiographs from a miniature schnauzer with chronic valvular disease and pulmonary edema. Note the tracheal elevation and perihilar pulmonary edema on the lateral view. On the dorsoventral view there is generalized cardiomegaly with a prominent left auricular appendage bulge at the 2-3 o'clock location. Click on image to see a larger view.
| Figure 1A | 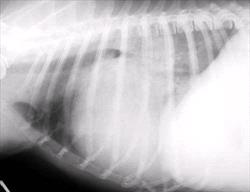
|
|
| |
| | Figure 1B | 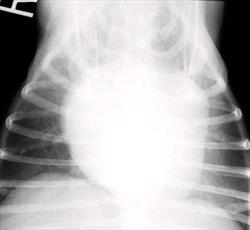
|
|
| |
|
Figure 2
Two-dimensional long axis echocardiogram from a dog with chronic valvular disease. The mitral valve is thickened and prolapses back into the left atrium during systole. Click on image to see a larger view.
Figure 3
Color-flow Doppler echocardiogram from a dog with chronic valvular disease. Note the mosaic pattern in the left atrium resulting from mitral regurgitation during systole. Click on image to see a larger view.