Introduction
The intraosseous (IO) space is a non-collapsible entry point into the systemic circulation that can essentially be thought of as the continuous and equivalent to the intravascular space. The IO route is often used when an intravenous route cannot be obtained. This is often the case in very small or young patients whose veins are too small to easily catheterize or in any age patient in a state of hemorrhage or dehydration when the peripheral veins are collapsed.
Manual vs. Automated Intraosseous Devices
The choice of manual IO devices varies with the age of the patient and ease of placement. In young animals with a soft bone cortex regular 18–25 ga hypodermic needles may be used. To facilitate passage of standard hypodermic needles the Near Needle Holder IO device (Near Manufacturing, Camrose, Alberta, Canada) was developed. Alternateively18–22 ga spinal needles may be preferred as they have a stylet to keep the needle lumen patent from blocking with cortical/medullary bone. In older patients, or patients in which it is difficult to traverse the cortex of the bone, Jamshidi bone marrow needles or commercial manual intraosseous needles may also be tried (i.e., Cook intraosseous infusion needle).
As a result of the difficulty in placing hand held devices in older patients, automated devices such as the Bone Intraosseous Gun (BIG) and EZIO have been developed and are now available to veterinarians. The EZIO is probably the more commonly used automated device in veterinary medicine and is available as 15 ga needle in lengths of 15, 25 and 45 mm.
What Are the Indications for IO Catheter Placement?
Any emergency situation where venous access cannot be rapidly obtained (i.e., trauma, cardiac arrest, status epilepticus, burn and shock patients) and young patients where vessels are too small to easily catheterize (IO devices often placed manually in these patients).
It should be noted that there is evidence to suggest the initial aspirate obtained from the IO catheter prior to flushing can be used for diagnostic studies including: Hemoglobin, hematocrit, glucose, BUN, creatinine, type and cross, blood gas and blood cultures.
What Are the Absolute Contraindications with IO Devices?
 Fracture or compromise (risk of pathologic fracture) in the target bone
Fracture or compromise (risk of pathologic fracture) in the target bone
 Compartment syndrome in the target extremity
Compartment syndrome in the target extremity
 Vascular injury in the target extremity (i.e., tibia is intact but there is a femoral fracture with significant soft tissue and vascular related injury in the proximal target limb)
Vascular injury in the target extremity (i.e., tibia is intact but there is a femoral fracture with significant soft tissue and vascular related injury in the proximal target limb)
 Acute infection at the insertion site
Acute infection at the insertion site
 Previous orthopedic surgery with hardware at the insertion site
Previous orthopedic surgery with hardware at the insertion site
 Recent failed IO attempt in same extremity (within 24–48 hours) - this leads to extravasation from the previous IO catheter insertion site
Recent failed IO attempt in same extremity (within 24–48 hours) - this leads to extravasation from the previous IO catheter insertion site
What Are the Relative Contraindications with IO Devices?
 Cellulitis or burns to the target extremity
Cellulitis or burns to the target extremity
 Sepsis or bacteremia (note this is systemic illness, not local infection of the target site which is an absolute contraindication)
Sepsis or bacteremia (note this is systemic illness, not local infection of the target site which is an absolute contraindication)
 Unable to palpate landmarks
Unable to palpate landmarks
What Are the Reported Possible Complications of Automated IO Use?
≤ 1 % in human studies
 Minor bleeding
Minor bleeding
 Fracture of the bone (rare)
Fracture of the bone (rare)
 Osteomyelitis (rare with appropriate sterile technique and removal of catheter within 24 hours) - human recommendations are to remove the device within 24 hours.
Osteomyelitis (rare with appropriate sterile technique and removal of catheter within 24 hours) - human recommendations are to remove the device within 24 hours.
 Pain (treated with lidocaine infusion - see below)
Pain (treated with lidocaine infusion - see below)
 Extravasation of fluid (can be up to 20% of cases). Risk factors include: incorrect needle placement, incorrect needle length and multiple punctures in the same bone. Osseous punctures can take 12–48 hours to clot; therefore, subsequent IO placement in the same bone should be avoided during that period.
Extravasation of fluid (can be up to 20% of cases). Risk factors include: incorrect needle placement, incorrect needle length and multiple punctures in the same bone. Osseous punctures can take 12–48 hours to clot; therefore, subsequent IO placement in the same bone should be avoided during that period.
 Compartment syndrome - a serious result of extravasation when fluid leaks out of the bone into the surrounding tissues (may increase with high pressure rates and prolonged infusion times) - can be a very serious complication.
Compartment syndrome - a serious result of extravasation when fluid leaks out of the bone into the surrounding tissues (may increase with high pressure rates and prolonged infusion times) - can be a very serious complication.
 Catheter dislodgement (i.e., as patient starts moving)
Catheter dislodgement (i.e., as patient starts moving)
 Fat embolism (demonstrated in dogs after 4 h of IO infusion although there was no clinical correlation [no change in PaO2 or shunt fraction])
Fat embolism (demonstrated in dogs after 4 h of IO infusion although there was no clinical correlation [no change in PaO2 or shunt fraction])
Note that obtaining gravity drip rates with a pressure bag does not mean malposition of the catheter, although the site should be verified for extravasation/compartment syndrome.
What Drugs Can Be Delivered Via the IO Route?
There is no significant difference in the peak effect of serum concentrations of commonly used emergency drugs (epinephrine, sodium bicarbonate, calcium chloride, hydroxyethyl starch and normal saline) when given via a central line, peripheral catheter or IO catheter. Most drugs given IV can be given IO including but not limited to blood products, synthetic colloids, hypotonic crystalloids, isotonic crystalloids, parenteral nutrition, opioids, antibiotics and most other IV medications (not all drugs have been evaluated by the IO route so it is impossible to say all drugs given IV can be given IO, but many have been investigated and found to be safe to administer IO). There are reports of tissue necrosis with extravasation of some drugs including hypertonic solutions (hypertonic saline, 10–50% dextrose), epinephrine, norepinephrine and sodium bicarbonate. It should be noted that extravasation of fluid is a complication of IO catheters (do not attempt to place an IO device in the same bone in which a previous attempt to place the device has failed). It should also be noted that although hypertonic solutions (hypertonic saline and 10–50% dextrose) can cause histologic evidence of bone marrow necrosis following their administration, the clinical significance of the necrosis has not established and most patients seem to do well following hypertonic IO fluid administration.
In summary, anything that can be given IV can likely be given IO, although consideration of the effects of extravasation must be considered.
How Quickly Can an Automated IO Device Be Placed and with What Success Rate?
A recent study in dehydrated children aged 3 months to 2 years found a 100% success rate to place an IO device within 5 minutes, compared to a 67% success rate for peripheral IV catheter placement. In pre-hospital ambulatory settings the success rate of IO devices is 84.8% in less than a minute. In states of pre-hospital cardiac arrest, successful vascular access was achieved 91% of the time with IO devices compared to 43% via a peripheral vein. With BIG devices cadaver studies show a success rate of 94%.
What Is the Risk of Infection with Automated IO Devices?
Risk of osteomyelitis attributed to IO cannulation in people is 0.6%. Most of these infections occurred during prolonged infusions or in situations of concurrent bacteremia at the time of IO catheter placement.
What Flow Rates Are Expected with IO Devices?
Within the diaphysis of the bone lies a central sinus composed of distensible endothelium that can accommodate a five-fold increase in volume. IO flow rates tend to vary with the size of the IO catheter used, the application of pressure bags/infusion devices, and the location the IO catheter is placed. The humerus tends to achieve the fastest flow rates in most studies when compared to the tibia. Swine studies have demonstrated that automated IO devices placed into the humerus can be given at 213 ml/min, the tibia 103 ml/min, and the femur 138 ml/min when saline was infused via a pressure bag. In people flow rates of 153 ml/min (humerus) vs. 165 ml/min (tibia) have been reported. When an 18 ga needle is used and fluid is administered by gravity flow rates of 35.6 ml/min have been reported. Higher IO flows rates are achieved with larger needles and rapid infusion devices/pressure bags (18 ga 205 ml/min; 16 ga 412 ml/min).
Is it Painful to Place an Automated IO Device?
Human studies report a score of 1–3 for pain on a visual analog scale of 1–10 (similar to insertion of an 18 g catheter into the vein) when the IO catheter is inserted/traverses the cortex. The discomfort reported with IO devices is likely less painful with automated devices. However, as the IO space contains nerve fibers that are sensitive to pressure, patients report a pain rating of 5 out of 10 during infusion of fluid. That said, several studies have shown the administration of preservative free 2% lidocaine into the IO space via the IO catheter can adequately manage the discomfort associated with infusions. In people, the recommended dose of lidocaine is 20–40 mg over 30 seconds delivered into the IO space. It is recommended to wait 60 seconds to achieve affect and then follow the lidocaine bolus with 10 ml of normal saline. It is reported that IO lidocaine may lose effectiveness after roughly 45 minutes and a repeated injection (not to exceed 3 mg/kg total) of lidocaine be administered in adults.
How to Place EZIO Automated IO catheter
 Landmarks are palpated to confirm the site of placement (common sites used include the medial tibia (see Figure 1) and lateral humerus (see Figure 2).
Landmarks are palpated to confirm the site of placement (common sites used include the medial tibia (see Figure 1) and lateral humerus (see Figure 2).
 Clip and aseptically prepare a 2 x 2" area of skin centered on the site of placement.
Clip and aseptically prepare a 2 x 2" area of skin centered on the site of placement.
 A small (3 mm) skin incision is made at the intended site of placement. This is done to prevent the skin from twisting around the catheter during placement.
A small (3 mm) skin incision is made at the intended site of placement. This is done to prevent the skin from twisting around the catheter during placement.
 The catheter with stylet is attached to the drill and seated against the bone, perpendicular to the surface of the bone. With the catheter seated against the bone, there must be at least 3–5 mm of clearance between the skin surface and the hub of the catheter. This is to allow room for the catheter to advance into the medullary cavity without compressing the overlying soft tissues.
The catheter with stylet is attached to the drill and seated against the bone, perpendicular to the surface of the bone. With the catheter seated against the bone, there must be at least 3–5 mm of clearance between the skin surface and the hub of the catheter. This is to allow room for the catheter to advance into the medullary cavity without compressing the overlying soft tissues.
 While maintaining gentle downward pressure, the drill is activated and the catheter advanced until a decrease in resistance is appreciated.
While maintaining gentle downward pressure, the drill is activated and the catheter advanced until a decrease in resistance is appreciated.
 Detach the drill and remove the stylet.
Detach the drill and remove the stylet.
 Marrow can potentially be aspirated at this time, though inability to aspirate marrow does not equate to failed placement. Marrow can potentially be used for blood-gas analysis.
Marrow can potentially be aspirated at this time, though inability to aspirate marrow does not equate to failed placement. Marrow can potentially be used for blood-gas analysis.
 Lidocaine (0.5 mg/kg) is administered IO over 30 seconds.
Lidocaine (0.5 mg/kg) is administered IO over 30 seconds.
 Flush with saline. During the flush, the limb adjacent the IO catheter site and along the opposite side of the bone (in case the opposite cortex was penetrated) is palpated to determine if any extravasation of fluid is appreciated. You'll find that there is more resistance to administering fluids IO compared to IV and this is normal.
Flush with saline. During the flush, the limb adjacent the IO catheter site and along the opposite side of the bone (in case the opposite cortex was penetrated) is palpated to determine if any extravasation of fluid is appreciated. You'll find that there is more resistance to administering fluids IO compared to IV and this is normal.
 If no extravasation is appreciated, a T-port can be connected, fluid therapy is initiated and catheter is bandaged in place.
If no extravasation is appreciated, a T-port can be connected, fluid therapy is initiated and catheter is bandaged in place.
| Figure 1 | 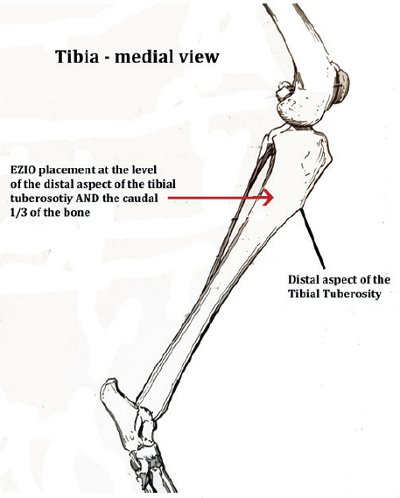
|
|
| |
| Figure 2 | 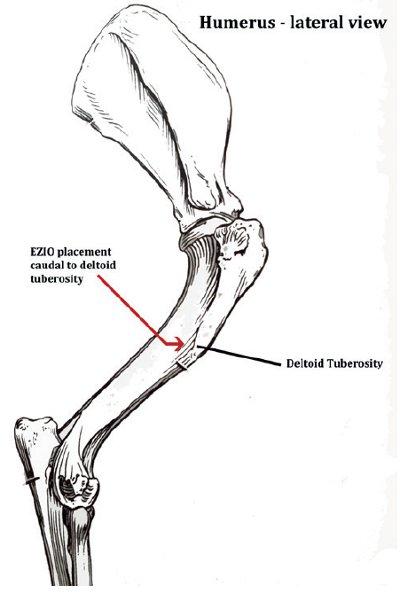
|
|
| |
Removal of the EZIO Catheter
 The T-port is removed.
The T-port is removed.
 A clean EZIO stylet of the appropriate size is placed inside the lumen of the catheter and locked in place.
A clean EZIO stylet of the appropriate size is placed inside the lumen of the catheter and locked in place.
 While twisting clockwise, the catheter is retracted.
While twisting clockwise, the catheter is retracted.
 After removal, inspect the catheter and stylet for bending or breakage.
After removal, inspect the catheter and stylet for bending or breakage.
 Although intended for single use only, the catheter and stylet can be resterilized for future use.
Although intended for single use only, the catheter and stylet can be resterilized for future use.
References
1. Anson AA. Vascular access in resuscitation. Anesthesiology. 2014;120:1015–1031.
2. Vizcarra C, Clum S. Intraosseous route as alternative access for infusion therapy. Journal of Infusion Nursing. 2010;33(3):162–174.