Hein P. Meyer, DVM, PhD, DECVIM
* The following lectures are included:
 Polyuria-polydipsia: a practical approach to a common clinical problem
Polyuria-polydipsia: a practical approach to a common clinical problem
 The treatment of renal diseases with ACE inhibitors: fact and fantasies
The treatment of renal diseases with ACE inhibitors: fact and fantasies
INTRODUCTION
Chronic renal failure (CRF) occurs in every dog and cat breed at any age, but older animals are more frequently affected than younger ones (Polzin, 1995). The prevalence in old cats (> 15 yrs) is 153:1000 and in younger animals 16:1000. In dogs these figures seem to be a little lower. The mean age of animals with CRF is approximately seven years. Hence, animals with CRF compose an important part of your patients. Their life expectancy is still poor, but with the improved knowledge and treatment modalities of CRF, considerable gains in quality of life and life expectancy income can be expected when sufficient time and care are given to these clients.
ETHIOPATHOGENESIS OF CRF
The primary lesion in the kidney may be glomerular, tubular, interstitial, vascular, or mixed (Table 1). If the primary lesion is severe enough and long-standing, progressive and often fatal kidney failure, affecting all renal compartments, may develop.
Table 1. Causes of Chronic Renal Failure
|
Glomerular |
Glomerulonephritis
(e.g., autoimmune) amyloidosis intoxications |
|
Tubular |
hypoxia (anaesthesia-surgery) intoxications
(e.g., heavy metals) viral infections
chronic subclinical leptospirosis |
|
Interstitial |
pyelonephritis focal nephritis |
|
Mixed |
many congenital disorders
(e.g., renal dysplasia, amyloidosis) |
|
Vascular |
diabetes mellitus (in man) |
In advanced cases, the primary lesion is often obscured by severe secondary changes, including tubulo-interstitial inflammation and fibrosis. Thus, the initiating cause may long be gone and often remains obscure to the clinician and pathologist. Pivotal in these events is the production of local growth factors which lead to interstitial fibrosis, and the increased production of endothelin and decreased production of bradykinin and NO, which lead to glomerulosclerosis. Intrarenal angiotensin II may be an important mediator in these changes (Gansevoort et al, 1994).
DIAGNOSIS OF CRF
First two definitions: azotaemia is referred to as the biochemical signs of a low GFR (i.e., increased plasma creatinine, urea, etc), whereas uraemia is defined as the clinical syndrome accompanying the accumulation of these substances (acute or chronic). So one can have azotaemia without uraemia. The symptoms of CRF with uraemia are mostly non-specific and include polyuria/polydipsia, dullness, poor appetite, weight loss, vomiting, a poor hair coat, and diarrhoea (Table 2). In severe cases signs may include muscle weakness, tremors, signs of uraemic encephalopathy, and a bad breath. Physical examination may be unremarkable, but may include signs of anaemia, stomatitis, neuromuscular changes, or signs of hypertension, such as a retinal detachment or a heart murmur.
Table 2. Main Findings during History and Physical Exam in Feline CRF*
|
Early Renal Failure |
Advanced Renal Failure |
|
History |
|
polyuria-polydipsia |
anorexia |
|
weight loss |
polyuria-polydipsia |
|
anorexia |
weight loss |
|
vomiting |
dysphagia |
|
lethargy |
lethargy |
|
Physical Exam |
|
thin |
thin |
|
periodontal disease |
dehydration |
|
poor hair coat |
pale mucous membranes |
|
cardiac murmur |
poor hair coat |
|
palpable thyroid |
small kidneys |
* In decreasing order of importance; from Elliott & Barber, 1998
Since signs and symptoms are often vague, CRF has to be included in many differential diagnoses, the most important one being polyuria/polydipsia (Table 3). If PU/PD is present, the initial diagnostic work-up should be directed towards excluding the various causes of this differential diagnosis.
Estimates of glomerular filtration rate (GFR) are most often used as an indicator of renal function/dysfunction. To date, the most specific biochemical method for this in daily practice still is the plasma (serum) creatinine concentration. Although plasma cystatin C has been mentioned to be superior (especially more sensitive), initial reports in dogs do not seem to endorse this (Almy et al, 2002).
Plasma creatinine is a function of the (in physiological situations rather stable) breakdown of muscle creatine on one hand, and excretion (through the gut and mainly via glomerular filtration) on the other hand. Thus, it is a function of (lean) body mass, and if a formula is applied which takes body weight into account, the reference range is much narrower (and thus, its diagnostic accuracy increases accordingly; Biewenga and van den Brom, 1981):
It cannot be over-emphasized that creatinine concentrations only rise after a substantial loss of glomerular function (Figure 1), so it is a rather insensitive measurement. The hyperbolic relationship between GFR and plasma creatinine and the individual variations in this relationship also imply that, in case of marginal increases in creatinine, it cannot be predicted whether GFR in the individual case is severely, or only mildly, impaired. A more sensitive indicator for measuring GFR, such as the plasma clearance of a radioactively labeled substance (like 99mTc-DTPA or EDTA), inulin, or iohexol, or alternatively, the endogenous or exogenous creatinine clearance, is indicated in such cases.
Plasma creatinine (in μmol/L): < 50 + (1.2 * body weight)
Table 3. Polyuria/Polydipsia--Its Differential Diagnosis
|
Frequent causes |
Rarer causes |
|
chronic renal failure |
hypercalcaemia (primary or neoplastic) |
|
diabetes mellitus |
central diabetes insipidus |
|
hyperadrenocorticism
(Cushing's syndrome) |
nephrogenic diabetes insipidus |
|
pyometra |
progestagens |
|
hyperthyroidism |
primary (psychogenic) polydipsia |
|
liver failure |
syndrome of inappropriate ADH release |
|
|
polycythaemia |
Although measuring plasma urea or BUN is still often performed for screening purposes for CRF, it is less specific than the plasma creatinine in getting an estimate of GFR, since plasma urea levels are, apart from GFR, also highly influenced by protein intake, catabolism, liver function, and tubular reabsorption. If, for example, a kidney diet with a lower protein content than the average diet is given, plasma urea decreases, whilst GFR is unaffected. Measuring creatinine and urea together in plasma may help to determine the cause of a decline in GFR (prerenal, renal, or postrenal): the urea:creatinine ratio in plasma (in mM and µM, respectively; ratio times 1000) in most cases of prerenal and postrenal azotaemia is > 150, and in renal causes of azotaemia < 100.
A low urine specific gravity (USG) has often been implicated as an early sign of CRF. However, healthy animals without kidney disease also may have a very low USG (van Vonderen et al, 1997) and PU/PD can be seen in many diseases other than CRF, so measuring USG is not very helpful as a screening test for CRF. It may, however, help to determine the cause of the declined GFR (low in renal causes of azotaemia, high in prerenal causes of azotaemia).
| Figure 1. | 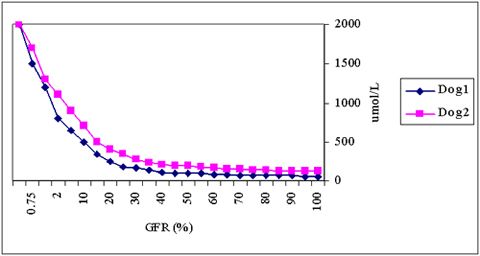
The hyperbolic relationship between GFR (X-axis) and plasma creatinine concentrations (Y-axis) with individual variations. |
|
| |
Once a decrease in GFR has been established, the cause of this decrease (prerenal, renal, or postrenal) has to be determined. A good history and physical exam may help (are there indications for fluid loss, cardiac problems, difficult micturition, dehydration, etc) as well as examination of a urinary sample (USG, protein concentration, sediment), and the ratio between urea and creatinine levels in plasma (see above). Assessment of dehydration by biochemical parameters is also useful (e.g., PCV, plasma osmolality, Na, albumin, total protein, etc), but each of these has its limitations. Once renal failure is suspected, measurement of PCV, reticulocytes, calcium, phosphate, sodium, potassium, and the acid-base balance may be performed to reveal the stage (acute or chronic) and severity of the kidney dysfunction. Very recently, determining the size of the parathyroids by ultrasonography has been shown to be helpful to differentiate between acute and chronic renal failure (Reusch et al, 2000). Determination of the urinary alkaline phosphatase : creatinine ratio (normally <10 x 103 U/μmol) and the plasma concentration of carbamylated haemoglobin may also be helpful to differentiate acute form chronic renal failure. The urinary AP:creatinine ratio is increased in acute renal failure as a result of the release of AP from damaged tubular cells in the urine (Heiene et al, 2001a), and plasma carbamylated haemoglobin concentrations are increased because of prolonged exposure to urea (Heiene et al, 2001 b). A urinary culture is essential in the course of the diagnostic work-up of CRF, because urinary tract infection often complicates the clinical picture of patients with CRF. Ideally, this should be repeated on a regular basis, but at least when the clinical picture changes considerably. Ultimately, histopathological examination of an ultrasound-guided kidney biopsy may reveal the primary cause and stage of kidney failure. However, if a case of 'normal CRF is suspected, the pathologist will only give 'chronic tubulointerstitial nephritis' as a result, which doesn't tell us anything new about the initial cause or the prognosis. So the indications for a performing a kidney biopsy are quite limited (Table 4).
Table 4. Indications for Taking an Ultrasound-Guided Renal Biopsy
 Differentiation acute from chronic renal failure
Differentiation acute from chronic renal failure
 Nephrotic syndrome
Nephrotic syndrome
 Suspicion of renal tumour
Suspicion of renal tumour
 Suspicion of pyelonephritis
Suspicion of pyelonephritis
 Renal haematuria
Renal haematuria
 Diagnosis of inherited renal disorder
Diagnosis of inherited renal disorder
 Suspicion of FIP or FeLV
Suspicion of FIP or FeLV
THERAPEUTIC CONSIDERATIONS IN CRF
Although the primary aim of this abstract is to cover the diagnostic work-up of patients with suspected renal failure, we would like to share some thoughts on the long-term management of these patients as well. Recent advances in this area have shown to increase both the quality of life and the life expectancy in these patients.
NUTRITIONAL MANAGEMENT
A protein-restricted kidney diet has been the corner stone in the treatment of CRF for many years. Although the rationale for protein restriction as main constituent of a kidney diet has been questioned (Finco et al, 1992), moderate protein restriction (of course, without bringing the patient in a state of amino acid deficiency) is still seen as one of the important measures for managing patients with CRF. Previously, deterioration of GFR and glomerular pathology were supposed to be halted by protein restriction. Although this has not been convincingly proved in the dog and cat, tubular and interstitial changes, which play a major role in the progression of CRF, may be inhibited by protein restriction. Moreover, the well-being of the patient improves if nitrogenous by-products of protein metabolism decrease. By adding soluble fibre to the diet, the excretion of nitrogen through the stool increases, further improving the well-being of your renal patients.
Apart from protein restriction, phosphate restriction is beneficial for patients with CRF. Either direct, or through secondary renal hyperparathyroidism, hyperphosphataemia leads to a further progression of kidney failure. Restriction of dietary phosphate intake has been shown to increase the life span and to stabilize GFR.
Sodium restriction and alkalinisation are other features of today's kidney diet.
Palatability of kidney diets has been a problem, but many manufacturers greatly improved palatability in the past few years. If patients refuse to eat a kidney diet, always remember that in many cases, there is a physical reason for this, e.g., gastritis, parodontitis, nausea, etc. Treating these underlying causes and making the diet more palatable (e.g., by heating the diet to body temperature) will help the patients eating better.
Increasing the dietary ω3: ω6 fatty acid ratio may be beneficial in decreasing glomerular pressure and changes in patients with CRF. This has been shown at least in experimental animals, but the idea has to be confirmed in larger studies with patients (Brown et al, 1998).
Very recently, a kidney diet with the features as discussed above (including moderate protein restriction, added soluble fibre, phosphate and sodium reduction, increased ω3: ω6 fatty acid ratio) has shown to increase survival time and reduce mortality and clinical signs compared to a maintenance type of food in canine patients with CRF (Figure 2).
PHARMACEUTICAL MANAGEMENT
Active Vitamin D (calcitriol) and phosphate binders
Hyperphosphataemia and a decreased activity of tubular 1-α hydroxylase lead to secondary renal hyperparathyroidism (SRH). If dietary phosphate restriction does not lead to normalization of plasma phosphate, phosphate binders may be added. Calcium carbonate may be used, but has the disadvantage of the risk of hypercalcaemia, which may further enhance CRF. A non-aluminum based phosphate binder, Renagel® has become available on the human market recently; we don't have experience with this new drug yet.
Even if plasma phosphate is normalized, SRH may develop. This may be prevented by the use of calcitriol. Nagode et al (1996), great advocates of the use of calcitriol in veterinary medicine, have shown that calcitriol in CRF leads to decreased plasma PTH levels. In this 'open' study, the quality of life of dogs and cats with CRF on calcitriol seemed also to improve.
| Figure 2. | 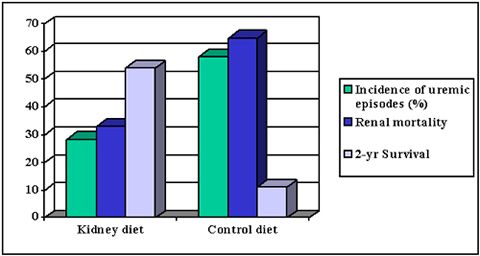
Comparison of a 'kidney diet' (Hill's Canine k/d) with a grocery-type diet in canine renal patients (all differences between kidney and control diet were statistically significant (p<0.05). Figure based on data from Jacob et al, 2000. |
|
| |
Angiotensin-converting enzyme inhibitors (ACE-I)
As mentioned under the etiopathogenesis of CRF, tubulo-interstitial changes are thought to play a pivotal role in the progression of renal failure from the initial insult to end-stage chronic renal failure. Thus, measures to slow the progression of these tubulo-interstitial changes potentially are of great benefit in these patients. Angiotensin II stimulates the production of several growth factors and vasoactive substances which enhance the local inflammatory response and subsequent fibrosis and sclerosis. Thus, ACE-I may inhibit these events by decreasing intrarenal ANG II concentrations. Many experimental studies in rats, and a few in dogs and cats (Brown et al 1993 and 2001) have shown a positive effect of ACE-I on the progression of CRF. Initial results of the use of the ACE-I enalapril in canine patients with CRF seem promising (Piek and Meyer, unpublished data). Apart from CRF per se, ACE-I have been proven of benefit in canine nephrotic syndrome, a specific subset of renal diseases of glomerular origin (Grauer et al, 2000)
Anti-emetics and antacids
Nausea, vomiting and subsequently anorexia are common features of CRF. Antiemetics, such as metoclopramide (Primperan®), could be given in such cases. Antacids, such as cimetidine or ranitidine may be even better choices, because metoclopramide has the theoretical disadvantage of stimulating gastrin release, which is already high in CRF. Gastric mucosa protectants, such as sucralfate, are very helpful as well; sucralfate (Ulcogant®) may also help to lower phosphate levels, because it is aluminum-based. Aluminum toxicity, a problem in humans with CRF undergoing haemodialysis, does not seem to be a problem in canine and feline CRF. It should be emphasized that the use of these drugs is based on anecdotal evidence, rather than on double-blind clinical studies.
Erythropoietin (EPO)
Anaemia in more advanced cases of CRF further debilitates these patients. Therefore, treatment of the anaemia may improve quality of life. In humans undergoing dialysis, treatment with human recombinant EPO is then often indicated. Many veterinarians also supply hr-EPO. Unfortunately, hr-EPO is expensive, it takes considerable time before the haematocrit rises, and often antibody production against hr-EPO develops later on. Haematocrit in canine patients using calcitriol often stabilizes, and sometimes even increases.
OTHER TYPES OF MANAGEMENT
Peritoneal or haemodialysis may be options to increase life span in CRF. However, veterinarians should be aware that these treatments are very labour-intensive and that improvement of the patients' quality of life should be the primary goal of any treatment. Regular (intravenous or subcutaneous) infusions have been reported to benefit patients with CRF as well. Ultimately, a kidney transplant is the therapy of choice. Results in cats are quite satisfactory. However, ethical considerations as to the donor animals (whether stray or not) should seriously be taken into account before this type of surgery is performed on healthy animals.
SUMMARY
In general, the diagnostic work-up of any clinical patient using the problem-oriented approach will bring you from the history and physical examination to the definition of a problem list, its differential diagnosis and its diagnostic procedures to rule out the various likely causes included in this list. The diagnostic protocol for patients with CRF specifically is best summarized by Figure 3 and Table 5. Modern therapeutic management of CRF offers our patients quite an increase in quality of life and survival.
| Figure 3. | 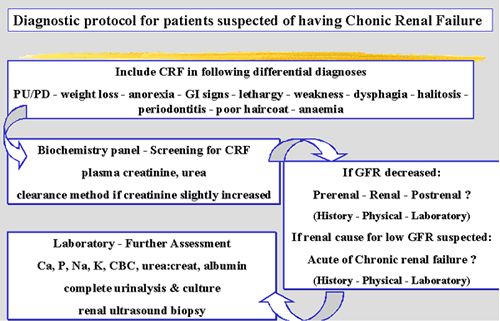
Diagnostic protocol for patients with a suspicion of CRF. |
|
| |
Table 5. Diagnostic Procedures to Consider in the Diagnostic Work-Up of Patients with Suspected Renal Disease
|
Plasma Biochemistry
creatinine, urea, urea:creatinine, carb-Hb, Ca, P, Na, K, osmolality, acid-base balance, albumin, total protein & electrophoresis, PTH, AT III, AVP |
|
Plasma Haematology
PCV (Ht), reticulocytes, leucocytes and differentiation |
|
Urinary Examination
physical properties, USG, osmolality, pH, Hb, glucose, protein:creatinine, AP: creatinine, urinary sediment, urinary culture (or culture pelvic fluid), fractional sodium excretion |
|
Imaging
ultrasonography (real-time, power-Doppler), retrograde and IV contrast, radioisotope renography, (plain X-rays?) |
|
Excretion Studies
inulin, radioisotopes (DTPA, EDTA, MAG III), iohexol |
|
Histopathology
HE stain, IFT, EM (cytology in most cases not useful) |
References
1. Almy FS, Christopher MM, King DP et al. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J Vet Int Med 2002; 16:45-51.
2. Biewenga WJ, van den Brom W. Assessment of glomerular filtration rate in the normal dog: Analysis of the 51 Cr-EDTA clearance and its relation to several endogenous parameters of glomerular filtration. Res Vet Sci 1981;30:152-7.
3. Brown SA, Walton CL, Crawford P et al. Long-term effects of antihypertensive regimens on renal hemodynamics and proteinuria. Kidney Int 1993;43:1210-8.
4. Brown SA, Finco DR, Brown CA Is there a role for dietary polyunsaturated fatty acid supplementation in canine renal disease? J Nutr 1998;128:2765S-2767S.
5. Brown SA, Brown CA, Jacobs G et al. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency. Am J Vet Res 2001;62:375-83.
6. Elliott J, Barber PJ. Feline chronic renal failure: Clinical findings in 80 cases diagnosed between 1992 and 1995. J Smal Anim Pract 1998; 39:78-85.
7. Finco DR, Brown SA, Crowell WA et al. Bennett SE Effects of dietary phosphorus and protein in dogs with chronic renal failure. Am J Vet Res 1992;5312:2264-7.
8. Gansevoort RT, de Zeeuw D, de Jong. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin-angiotensin system? Kidney Int 1994;45:861-7.
9. Grauer GF, Greco DS, Getzy DM et al. Effects of enalapril versus placebo as a treatment for canine idiopathic glomerulonephritis. J Vet Int Med 2000;14:526-33
10. Heiene R, Moe L, Molmen G. Calculation of urinary enzyme excretion, with renal structure and function in dogs with pyometra. Res Vet Sci 2001a;70:129-37.
11. Heiene R Vulliet R, Williams RL et al. Use of capillary electrophoresis to quantitate carbamylated hemoglobin concentrations in dogs with renal failure. Am J Vet Res 2001b;62:1302-6.
12. Jacob F, Polzin DJ, Allen T et al. Relationship of diet to uremic morbidity in dogs. J Vet Int Med 2000; 14: 350
13. Nagode LA, Chew DJ, Podell M. Benefits of calcitriol therapy and serum phosphorus control in dogs and cats with chronic renal failure. Both are essential to prevent of suppress toxic hyperparathyroidism. Vet Clin North Am Small Anim Pract 1996;266:1293-330.
14. Polzin DJ. In: Textbook of Veterinary Internal Medicine, S.J. Ettinger (ed.) 1995, 1734-58.
15. Reusch CE, Tomsa K, Zimmer C et al. Ultrasonography of the parathyroid gland as an aid in differentiation of acute and chronic renal failure in dogs. J Am Vet Med Assoc 2000;217:1849-52.
16. van Vonderen IK, Kooistra HS, Rijnberk A. Intra-and inter-individual variation in urine osmolality and urine specific gravity in healthy pet dogs of various ages. J Vet Int Med 1997;111:30-5.