Preliminary Evaluation of a Portable Clinical Analyzer to Determine Blood Gas and Acid-Base Parameters in Manatees (Trichechus manatus)
Abstract
This study was conducted to validate the i-STAT machine (Heska Corp., Fort Collins, CO, USA) for analyzing manatee blood gases and acid-base parameters. Blood was collected from captive (n=2) and wild (n=12) manatees to determine preliminary reference ranges for the i-STAT. Values from captive animals were compared to standard blood gas, clinical chemistry, and complete blood cell count machines. Also, blood values were compared between captive and wild manatees. The i-STAT machine reported sodium (140.0±2.92) and chloride (97.80±2.17) as lower and higher, respectively, than the reference machines. Additionally, wild manatees had higher values for lactate (14.84±3.42), glucose (90.50±22.39) and a lower pH (7.08±0.14) than captive manatees.
Introduction
Capture and veterinary care of manatees frequently occurs in the field in relatively remote areas. The quick evaluation of blood gas parameters and acid-base status in manatees may aid in treatment of these animals in the field and during rehabilitation at oceanaria. Also, detection of significant respiratory or metabolic changes associated with capture may be used to assess and potentially modify capture designs to minimize physiologic stress. The recent development of portable clinical analyzers, such as the i-STAT (Heska Corp., Fort Collins, CO, USA), have allowed point-of-care analysis in settings where this was previously impossible. The i-STAT has been validated for use in humans, domestic animal species, and northern elephant seals (Mirounga angustirostris) in field and acute-care settings.1-4 The i-STAT is a hand-held, self-powered, portable unit that utilizes a small amount of heparinized, whole blood instead of using serum or plasma. Since clot formation and separation are not required, results are available in less than 5 minutes from venipuncture. However, the i-STAT’s methodology must be validated and compared to reference methodologies in each species to be examined prior to use. The i-STAT uses a different methodology, potentiometry and measurement of ionic charge, to determine analyte values than do standardized laboratory analyzers. The differences in sample processing and methodology between the i-STAT and laboratory machines can result in clinically significant differences in reported analyte concentration and reference intervals. This study was conducted to validate the i-STAT machine for use in manatees.
Materials and Methods
Monthly blood samples were collected voluntarily, without restraint, from two trained, captive manatees held at Mote Marine Laboratory from the lateral brachial vascular plexus into lithium heparinized blood tubes. Blood samples were placed on ice and transported to Sarasota Memorial Hospital. At the hospital, samples were analyzed at the same time using the i-STAT and a standard blood gas machine. Samples were also processed by a standard chemistry and complete blood cell count machine within 1–3 h of blood collection. Additionally, blood samples were collected from twelve wild manatees captured during health assessment and satellite tagging research projects conducted by Florida Fish and Wildlife Conservation Commission and the Sirenia Project during 2003 and 2004. Animals were caught in the water by circle net and processed on a boat or moved to land. Blood samples were taken at the beginning of the capture period (Pre) and at least 20 minutes or more after the initial sample was taken prior to release (Post). Blood samples were analyzed in the field using the i-STAT as soon as possible after sampling. The i-STAT cartridges used were the CG4+, EC8+ and Crea cartridges. The following parameters were tested per cartridge: CG4+ = lactate, total carbon dioxide content (TCO2), hydrogen ion concentration (pH), partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2), bicarbonate ion concentration (HCO3), base excess (BE), hemoglobin saturation with oxygen (SO2); EC8+ = glucose (Glu), blood urea nitrogen (BUN), sodium (Na), potassium (K), chloride (Cl), anion gap (AnGap), hematocrit (Hct), hemoglobin (Hb), pH, PCO2, HCO3, BE; Crea = creatinine (Crea). Means and standard deviations were calculated for all values measured. Means were compared between the i-STAT and standard blood gas, clinical chemistry and complete blood cell count machines at Sarasota Memorial Hospital for the captive manatees using one-way analysis of variance (ANOVA) with Bonferroni-adjusted post hoc comparisons. For wild manatees, means from Pre and Post samples for each i-STAT cartridge were compared using a paired t-test. Additionally, i-STAT variables were compared between captive animals and wild animals using ANOVA with Bonferroni-adjusted post hoc comparisons. If normality or equal variance was violated, variables were analyzed using Kruskal-Wallis tests. All statistical calculations were performed with the SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). For all analyses, values of p≤0.05 were considered significant.
Results
Preliminary i-STAT reference ranges for certain blood parameters in captive manatees and wild manatees were determined (Table 1). For captive manatees, the mean time between the blood being sampled and being processed in the i-STAT machine was 52.5±11.5 minutes. For the wild manatees, mean processing time out of the water was 66.08±11.7 minutes. Mean time between Pre and Post samples being taken was 34.42±12.5 minutes. Mean time between the blood being sampled and being processed in the i-STAT machine was 20.00±12.0 minutes. For captive manatees, the only significant difference in parameters measured by the i-STAT machine and the standard blood machines were found in Na, Cl, Hb and Crea (Table 2). For the wild manatees, the only significant differences between Pre and Post samples were pH, Glucose, and Na (Table 3). Lastly, between the captive and wild manatees, there were significant differences in Lactate, pH, Glu, BUN, Na, and Cl. (Table 4)
Table 1. Preliminary i-STAT values for specific blood parameters in captive and wild manatees
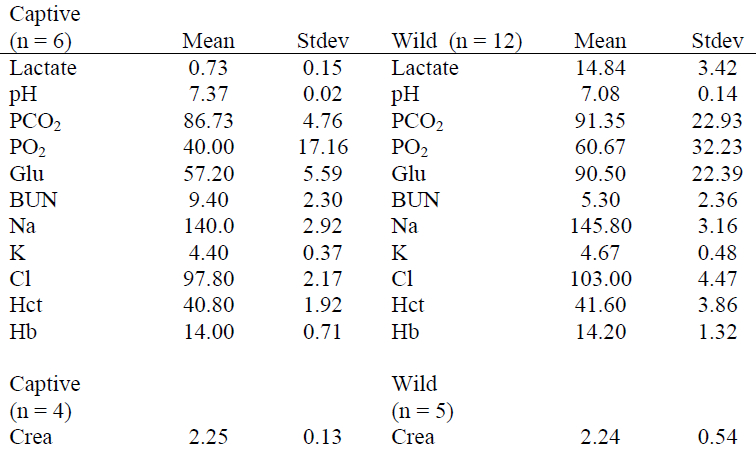
Table 2. Preliminary comparison of i-STAT values with standardized reference methodologies in repeated blood samples from trained captive manatees
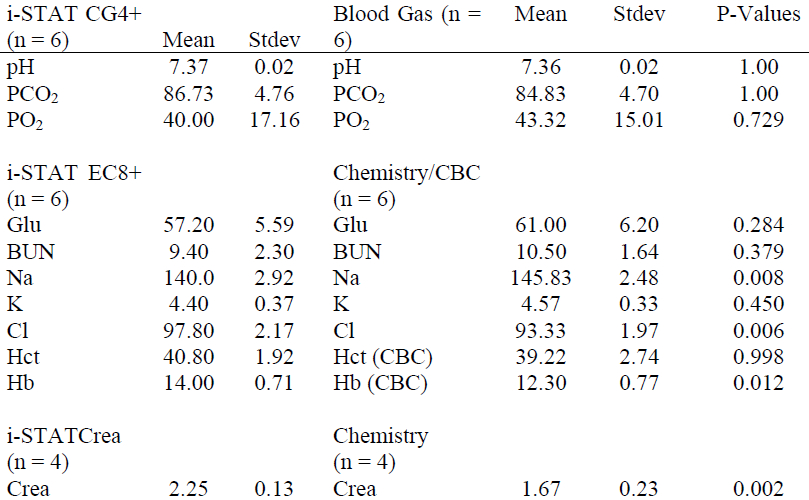
Table 3. Preliminary comparison of i-STAT values collected at the beginning of capture (Pre) and just prior to release (Post) for wild manatees
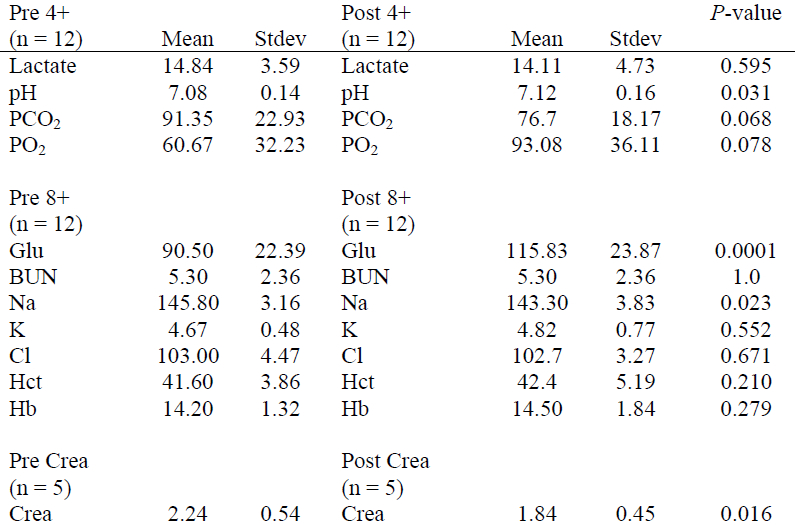
Table 4. Mean differences between blood values measured using the i-STAT in trained captive vs. wild captured manatees
Discussion
The values generated by the i-STAT for TCO2, HCO3, BE, and SO2 were calculated from human normograms based on temperature, pH, and hemoglobin affinity. These variables would be markedly different between humans and marine mammals. For this reason, these values were not analyzed in this study. Manatees were bled from a mixed arteriovenous plexus where shunting of blood may occur. Therefore, there may be a dramatic difference in pO2 and pCO2 simply because of the difference in arterial, venous or mixed blood values. We hope in the future to gather enough samples to separate arterial samples from venous samples based on pO2 to generate reference intervals for both arterial and venous samples. Currently, however, discrepancies in pO2 and pCO2 cannot be evaluated at this time due to low sample numbers.
There currently are not enough samples collected to generate an accurate reference interval, and the one presented is only preliminary. The low animal numbers also preclude final determination of statistical relevance. However, some values are evidently different in the samples and are noteworthy. A significant decrease in sodium concentration and an increase in chloride concentration are present when measured by the i-STAT as opposed to the clinical chemistry machine. This is consistent with previous reports in pinnipeds and dogs using alternate standardized instruments.1,2 This indicates that there is likely a significant difference in methods between the two machines and this difference should be considered when testing other marine mammals.
For wild animals there was a significant increase in glucose concentration when comparing initial values with release values in the same animal. In other species, this increase may be caused by endogenous glucocorticoid (cortisol/corticosterone) release which has not been well documented in manatees during capture operations. Additional significant differences for pH, Na, and Crea were noted between Pre and Post samples but these differences may not be biologically significant for the parameters tested. An increase in sample size will help determine if there are really significant differences in these parameters in the future.
Lastly, between the captive and wild animals there were several significant differences in certain blood values. In wild-caught animals, lactate and glucose were both increased. The increase in lactate is expected from an animal that is undergoing anaerobic glycolysis secondary to struggling during capture. The increased glucose may also be associated with capture stress. The decreased pH as compared to captive animals was striking in some individuals. This apparent acidosis may be secondary to the sample being more venous in nature or it could be secondary to a possible dive reflex exhibited by captured manatees. Continued investigation into the mechanism behind this acidosis is ongoing. In conclusion, the i-STAT appears to be a useful tool for clinically assessing manatees. However, the i-STAT has some values that are significantly different from standard reference machines, and i-STAT values should not be directly compared to standard blood gas or clinical chemistry machines but compared to i-STAT reference ranges established for each new species tested.
Literature Cited
1. Larsen, R.S., M. Haulena, C.B. Grindem, and F.M. Gulland. 2002. Blood values of juvenile Northern elephant seals (Mirounga angustirostris) obtained using a portable clinical analyzer. Veterinary Clinical Pathology. 31(3): 106–10.
2. Looney, A.L., J. Ludders, H.N. Erb, R. Gleed, and P. Moon. 1998. Use of a handheld device for analysis of blood electrolyte concentrations and blood gas partial pressures in dogs and horses. Journal of the American Veterinary Medical Association. 213: 526–531.
3. Papadea, C., J. Foster, S. Grant, S.A. Ballard, J.C. Cate, W.M. Southgate, and D.M. Purhit. 2002. Evaluation of the i-STAT portable clinical analyzer for point of care blood testing in the intensive care units of a university children’s hospital. Ann Clin Lab Sci. 32(3):231–243.
4. Verwaerde, P., C. Malet, M. Lagente, F. de la Farge, and J.P. Braun. 2002. The accuracy of the i-STAT portable analyzer for measuring blood gases and pH in whole-blood samples from dogs. Res Vet Sci. 73(1):71–5.