Pharmacokinetics of Florfenicol After a Single Intramuscular Dose in White-Spotted Bamboo Sharks (Chiloscyllium plagiosum)
Abstract
Florfenicol is an often-used antibiotic in fish medicine, however little is known of its pharmacokinetics in these species. Pharmacokinetic studies in Atlantic salmon (Salmo salar) demonstrate a high bioavailability, a significant degree of tissue penetration, and high efficacy.1-3 However, florfenicol pharmacokinetic data in red pacu (Colossoma brachypomus) indicated rapid elimination, necessitating high dosages (estimated at 20–30 mg/kg) administered every 24 h.4 The purpose of this study was to evaluate the pharmacokinetic properties of florfenicol in shark species, represented here by the white-spotted bamboo shark (Chiloscyllium plagiosum), and to quantify the concentration of florfenicol (bioavailability) in plasma and cerebrospinal fluid (CSF) after a single intramuscular injection in determination of its efficacy and longevity in treating a bacterial meningitis. This study is the only known report on florfenicol pharmacokinetics in a shark species and the only known report on florfenicol pharmacokinetics in CSF of a fish species.
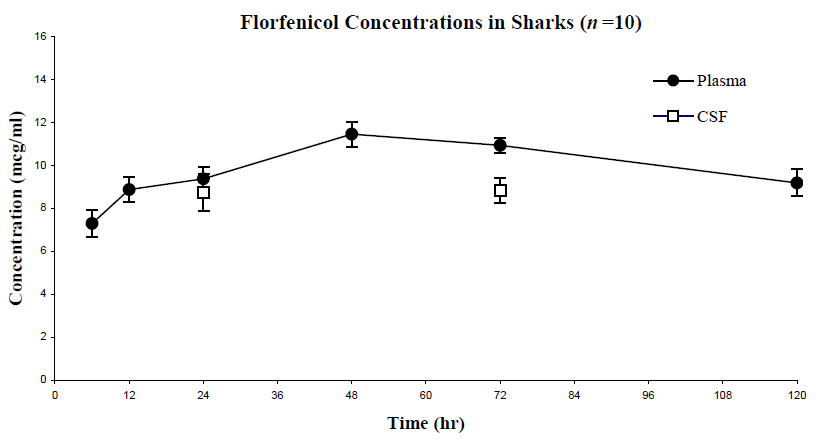
A pilot study was performed with three sharks to determine an adequate therapeutic dose of florfenicol based on the approximate peak and duration of a single dose given intramuscularly. A therapeutic dose was defined in this study as one that provided plasma levels within the range of 4–8 µg/ml, based on the mean minimum MIC for the most common pathogenic organisms using NCCLS breakpoints, maintained throughout the dosing interval.5 Plasma concentrations peaked at around 36 h. This time was used to determine CSF sampling times for the study in order to minimize anesthesia time and CSF loss. From this data, the study dosage of 40 mg/kg was determined: a dose that maintained plasma levels above the MIC mark of 4–8 µg/ml and appeared “safe” enough to allow for accumulation when administering multiple doses. A 72-h dosing interval was expected. Ten adult white-spotted bamboo sharks (five males and five females) were used for the pharmacokinetic study of florfenicol. On the first day of the study, each shark was anesthetized in 50 ppm tricaine methanesulfonate (MS-222®, Argent Chemical Laboratories, Redmond, WA, USA), examined, and weighed. “Pretreatment” (0 h) plasma and CSF samples were collected, and each shark then received a single dose of florfenicol (Nuflor®, Schering-Plough, Union, NJ, USA) at 40 mg/kg, which was administered deep intramuscularly (primarily delivered into white muscle) craniolateral to the dorsal fin. Blood samples were collected under manual restraint, except when CSF was collected, in which case anesthesia was used. Blood samples were obtained from the caudal vein at 0, 6, 12, 24, 48, 72, and 120 h post-injection via a 22-G, 1-inch needle. CSF samples were collected under anesthesia at 0, 24, and 72 h post-florfenicol administration via a 22-G, 1-inch needle inserted at the soft depression on top of their head, medial and slightly caudal to the eyes. Quantitative analysis was performed on the samples through high-performance liquid chromatography (HPLC) to determine florfenicol concentrations (Figure 1). Florfenicol plasma levels appeared to peak at around 48 h, and were maintained well above the target MIC of 6 µg/ml for 120 h. CSF concentrations mirrored plasma concentrations, also surpassing the target MIC for a minimum of 72 h. These results warrant the use of florfenicol as a primary choice in the treatment of systemic and CNS infections in sharks and possibly other fish species.
Figure 1. Florfenicol concentrations versus time
Acknowledgments
The authors wish to thank the aquarists at the Henry Doorly Zoo including curator Sean Putney, the veterinary hospital staff including Gerald Huckmeier, Nicole Linafelter, Andrew DeJong, Jon Timmerman, and the staff at the North Carolina State University Clinical Pharmacology Laboratory for their assistance.
Literature Cited
1. Horsberg T.E., Hoff K.A., and R. Nordmo. 1996. Pharmacokinetics of florfenicol and its metabolite florfenicol amine in Atlantic salmon. J. Aquatic Anim. Health. 8: 292–301.
2. Horsberg T.E., Martinsen B., and K.J. Varma. 1994 The disposition of 14C-florfenicol in Atlantic salmon (Salmo salar). Aquaculture. 122: 97–106.
3. Martinsen B., Horsberg T.E., Varma K.J., and R. Sams. 1993. Single dose pharmacokinetic study of florfenicol in Atlantic salmon (Salmo salar) in seawater at 11°C. Aquaculture. 112: 1–11.
4. Lewbart G.A., Papich M., and D. Whitt-Smith. 1999. Pharmacokinetics of florfenicol in the red pacu (Colossoma brachypomum) after single dose intramuscular administration. Proc. Int. Assoc. for Aquatic Animal Med. Vol. 30, Pp. 123–125.
5. NCCLS. 2002. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard Second Edition. NCCLS document M31–A2. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA.