Abstract
Hematologic and serum biochemical values were measured for 18 free-ranging fossas (Cryptoprocta ferox). Fecal samples and ectoparasites were collected for parasite identification. Serologic testing was performed to assess exposure to common canine and feline pathogens (canine adenovirus, canine distemper, canine herpesvirus, canine parvovirus, feline calicivirus, feline enteric coronavirus, feline herpesvirus, Dirofilaria immitis, and Toxoplasma gondii). Serologic assays were also performed for free-ranging domestic dogs (Canis familiaris) (n=35) and cats (Felis catus) (n=32). Parasites of the free-ranging domestic carnivores were identified.
Introduction
Madagascar is home to eight species of endemic herpestid and viverrid carnivores. These predators are increasingly threatened by habitat destruction, impact of introduced species and declining prey populations. Baseline health data are needed to provide veterinary care for captive Malagasy carnivores and for future use in monitoring the health of wild populations. With knowledge of the parasites and diseases that plague the wild carnivores, it may be possible to prevent the spread of disease or to intervene in the case of an outbreak. Data regarding the prevalence of infectious diseases in the domestic carnivore population will be important for future monitoring and population risk assessment. They may also be a significant factor when determining policy regarding human activities within park boundaries.
Methods
Free-ranging fossas (Cryptoprocta ferox) were sampled in three regions of dry deciduous forest in western Madagascar (Ankarana, Ankarafantsika, and Kirindy Mitea) during the dry season (May–December) of 2000 and 2001. Animals were immobilized as part of an ongoing ecologic study. Fossas were trapped in cage traps, and anesthetized with tiletamine and zolazepam (Telazol, Fort Dodge Animal Health, Fort Dodge, IA, USA) dosed at approximately 10 mg/kg via blowpipe and dart (Pneu-dart, Pneu-Dart, Inc., Williamsport, PA, USA).
Blood, feces, and ectoparasites were collected. Total white blood cell counts were performed (Unopette 5853, Becton Dickinson, Franklin Lakes, NJ, USA). Blood smears stained with Wright-Giemsa stain were examined for parasites and differential leukocyte counts. Blood was centrifuged for 15 minutes at 3100 rpm. Packed cell volume (PCV) was determined and serum was frozen in liquid nitrogen. Plasma total solids were measured by refractometer. Fecal samples were preserved in sodium acetic acid formalin (Protocol SAF fixative vials, Fisher Healthcare, Swedesboro, NJ, USA). Ectoparasites were preserved in 70% ethanol. Serum biochemical analyses, serologic assays for infectious agents and parasite analyses were performed at the New York State Animal Health Diagnostic Laboratory (Ithaca, NY, USA).
Results
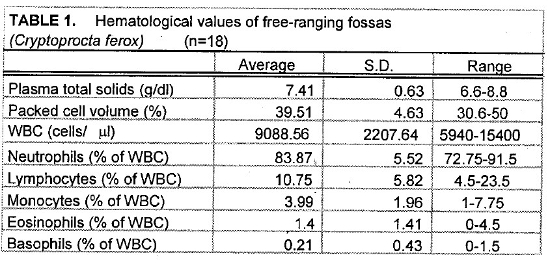
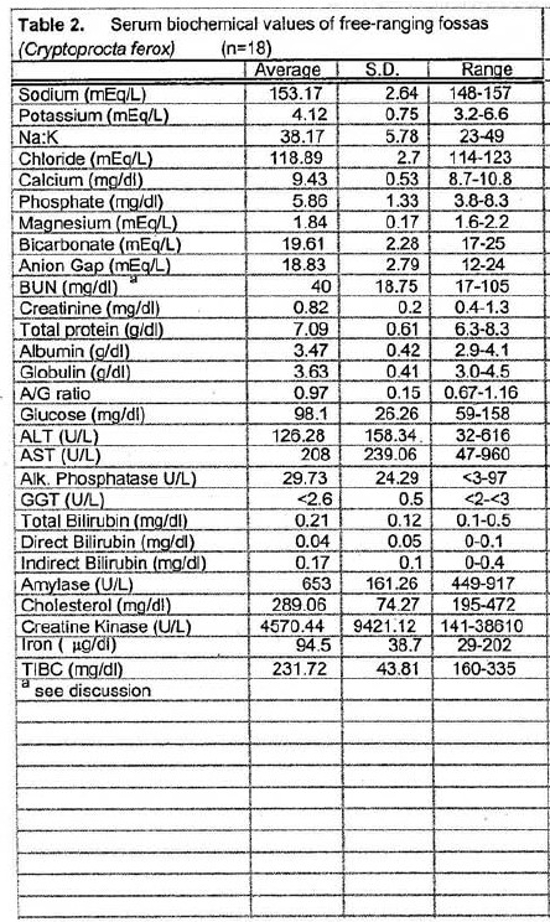
Tables 1 and 2 present the hematologic and serum biochemical analysis results for 18 fossas.
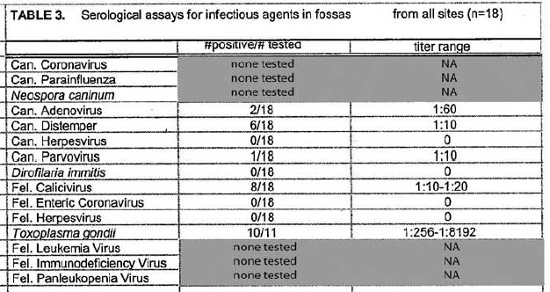
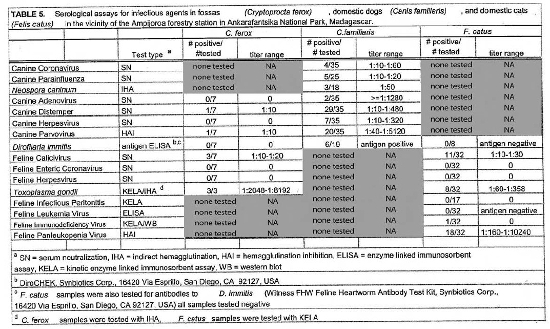
Serologic assays for canine and feline infectious agents performed on samples from sympatric fossas, dogs, and cats in the vicinity of the Ampijoroa forestry station are presented in Table 5. Results for all fossas sampled at the three sites are presented in Table 3. Positive titers were found in the dog population for all of the infectious agents tested for. Eighty-three percent of the dog population had positive titers for canine distemper virus and 57% for canine parvovirus. Feline panleukopenia virus antibodies were present in 56% of the cat population. Low titers to feline calicivirus were present in 34% of the cat population. Toxoplasma gondii antibodies were detected in 25% of the cats sampled. There was no serologic evidence of the presence of feline leukemia virus, feline enteric coronavirus, feline infectious peritonitis, or feline herpesvirus. Fossas sampled at Ampijoroa showed low or no serologic evidence of exposure to most of the canine and feline infectious agents. Low positive titers to canine distemper and canine parvovirus were each found in one individual. Feline calicivirus antibodies were present at a low level in 43% of the fossas. Ninety-one percent (10/11) of fossas from all study sites had high positive T. gondii titers.
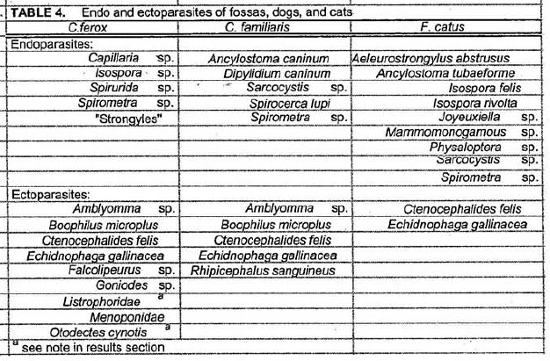
Endo and ectoparasites from fossas, dogs and cats are presented in Table 4. Most animals were infested with Ctenocephalides felis and Echidnophaga gallinacea. The fossas may have acquired the E. gallinacea from the chickens used as bait. Endoparasites isolated from fossa fecal samples included Isospora sp., Capillaria sp., Spirurida sp., Spirometra sp., and “strongyles.” Listrophoridae and Otodectes cynotis mites were found in fossa feces but it is not known whether these were ingested fossa ectoparasites or parasites of prey items. No blood parasites were detected in the fossa blood smears but several dogs had positive antigen tests for Dirofilaria immitis and microfilariae were observed in the blood smears of two dogs. Haemobartonella felis has previously been reported in a cat in Madagascar and H. felis like organisms were seen in blood smears of some (8/16) cats.5
Discussion
Hematologic and biochemical parameters for the fossas in this study are similar to the normal ranges for domestic dogs and cats. Compared to normal values for domestic dogs, the fossas’ mean leukocyte values demonstrate a mild leukocytosis with mature neutrophilia, lymphopenia, and eosinopenia—characteristics of a stress leukogram. However, there is no monocytosis as is commonly seen in a canine stress leukogram.
The slightly high blood urea nitrogen (BUN) and creatinine levels seen in the fossas of this study are not surprising. These animals are adapted to a seasonally arid climate and they were sampled during the dry season when their entire water intake comes from their prey items. One animal in this sample set had an unusually high BUN (105 mg/dl), more than twice the value for any other animal. This animal’s PCV and plasma total solids were similar to the mean values suggesting that pre-renal azotemia due to dehydration was unlikely. Renal compromise also seemed unlikely since this animal appeared to be in good condition during captures 1 year before and 1 year after the high BUN blood sample (no blood samples were obtained at those times). If this high value is eliminated from the sample set, the mean BUN value is 36.18 mg/dl (SD= 9.70, range 17–50). Elevated creatine kinase (CK) seen in several samples was likely due to trauma from struggling in the trap and muscle trauma induced by darting. The animal with the highest CK (38610 U/L) was a lactating female who sustained a skin laceration while in the trap. Elevated aspartate transferase (AST) activity may also have been associated with exertion and muscle trauma.
There have been few published reports of infectious disease in free-ranging Malagasy carnivores and their susceptibility to canine and feline pathogens is unknown. Although the assays used in this study have not been validated for C. ferox, the findings may still be cautiously interpreted to assess exposure to the infectious agents. Confounding factors may include nonspecific reactivity, failure of C. ferox to mount a detectable immune response to a specific pathogen, or high mortality among exposed animals. Members of the Viverridae are thought to be susceptible to canine distemper and a parvovirus (not isolated) has been implicated in some disease outbreaks.1,6 Six of 18 (33%) fossas in this study had low positive canine distemper antibody titers but only one of 18 animals had a low positive canine parvovirus titer. The low positive results could be non-specific reactions, considering the high values seen in other species where infection has been documented. Both diseases were prevalent among the domestic dog populations including individuals with high titers suggestive of recent exposure or infection.
Toxoplasmosis is a disease of particular concern. It is thought that cats, the only definitive hosts for T. gondii, arrived in Madagascar with the first human colonists about 2000 years ago. Species that have evolved in places without cats are often particularly vulnerable to T. gondii. Australian marsupials and Malagasy lemurs infected with T. gondii frequently succumb to fatal toxoplasmosis. As human populations have penetrated further into wild areas, cats have also invaded. The impact of T. gondii on wild populations of lemurs and other Malagasy wildlife has not been assessed. As top predators, fossas are likely to become infected with T. gondii through consumption of various secondary hosts. Two cases of clinical disease attributed to T. gondii have occurred in captive Malagasy carnivores: one C. ferox (Gary West, personal communication) and one Galidictis vittata (Note: It is likely that the animal was actually G. fasciata or grandidieri).4 High antibody titers to T. gondii among fossas in remote areas are alarming.
Spirocerca lupi was present among the dogs of this study and it has previously been reported as the cause of death for 57.5% of lemurs that died in 1 year at the National Zoo in Madagascar.2 The susceptibility of Malagasy carnivores to this parasite remains unknown. Spirurida sp. eggs were isolated from the feces of fossas but they could not be identified to the species level.
Tiletamine/zolazepam provided smooth anesthesia with rapid onset of action, but the recovery period was prolonged (more than 10 hours in most cases). Anesthetic monitoring parameters (heart rate, respiratory rate, and SpO2) remained within acceptable limits and the same animals were often recaptured months later having suffered no apparent detrimental effects from the previous immobilization. Nevertheless, prolonged recovery time is undesirable because it interferes with normal activities, increases the amount of time spent in a stressful environment and because other factors such as the anesthetic’s effect on blood pressure are unknown. Similarly undesirable anesthetic results have been reported with a xylazine and ketamine combination.3 The authors are currently transitioning to the use of a medetomidine (Domitor, Pfizer, Inc., New York, NY, USA) and ketamine (Ketaset, Fort Dodge Laboratories, Fort Dodge, IA, USA) combination which has been used with good results in captive fossas (Donald Janssen and Gregory Levens, personal communications).
Acknowledgments
The authors thank the Pittsburgh Zoo Conservation Fund and Earthwatch Institute for their generous support. We also thank Dr. Stuart Pimm for his guidance; Stephanie L. Schaaf and Peter E. Ziegler for analyzing the parasite samples; and Martel Jaonina, Elizabeth Boakes, Shaun Dunn, and all of the Earthwatch volunteers who participated in the collection of samples.
Literature Cited
1. Barker IK, Parrish CR. Parvovirus infections. In: Williams ES, Barker IK, eds. Infectious Diseases of Wild Mammals. Ames, IA: Iowa State University Press; 2001:131–146.
2. Blancou JM, Albignac R. Note sur l’infestation des Lemuriens malagaches par Spirocerca lupi (Rudolphi, 1809). Revue d’Elévage et de Medecine Veterinaire des Pays Tropicaux. 1976;29(2):127–130.
3. Hawkins CE. Behaviour and ecology of the fossa, Cryptoprocta ferox (Carnivora: Viverridae) in a dry deciduous forest, western Madagascar. Ph.D. Dissertation. University of Aberdeen, Aberdeen, Scotland. 1998.
4. Uilenberg G. Quelques protozoaires parasites de mammifères sauvages à Madagascar. Annales de Parasitologie (Paris) 1970;45(2):183–194.
5. Uilenberg G, Lapeire C. Existence de l’anémie infectieuse féline (épérythrozoonose du chat) à Madagascar. Revue d’Elévage et de Medecine Veterinaire des Pays Tropicaux. 1967;20(2):355–357.
6. Williams ES. Canine distemper. In: Williams ES, Barker IK, eds. Infectious Diseases of Wild Mammals. Ames, IA: Iowa State University Press; 2001:50–59.