Gamma Interferon Enzyme Immunoassays and Their Use in the Investigation of Tuberculosis in a Western Lowland Gorilla
Helen E. McCracken, BSc (Vet), BVSc, MVS
Abstract
Tuberculosis (TB) in non-human primates, caused by Mycobacterium tuberculosis or Mycobacterium bovis, is a disease of great concern in zoos due to its capacity for insidious spread to other collection animals, free-ranging animals in zoo grounds, and to human contacts. The majority of TB infections are controlled by the host’s immune defences and remain latent; however, some such infections progress to active and contagious disease. It is very important, therefore, to have a sensitive and specific diagnostic test to identify latent cases so that further spread of disease may be prevented by either treatment or culling of these individuals.4 Over the past decade, gamma interferon (IFN-γ) enzyme immunoassays (EIA) have been developed for rapid in vitro diagnosis of TB in several species, including humans and non-human primates. This paper reviews the relative merits of these assays and other currently available TB diagnostic techniques. Details are presented of the investigation of TB in a western lowland gorilla at Melbourne Zoo, Australia, demonstrating the application of these assays.
The “gold standard” for TB diagnosis is isolation of the mycobacterial organism by culture.1,5 This process, however, can take up to 8 weeks, and in 10–20% of human TB cases the organism is not successfully isolated.1 Furthermore, bacteria can only be cultured from cases of active disease, and the test is therefore of no value in the diagnosis of latent TB.1
The screening test for both latent and active TB used for many years in both humans and non-human primates is the tuberculin skin test (TST). Intradermal injection of tuberculin purified protein derivative (PPD) will induce a cell-mediated immune (CMI) response in infected individuals, producing induration at the injection site, measured at 72 hours.5 Although this test has been used widely in humans for over a century, it is subject to considerable variation and other limitations. Inconsistencies and errors in the administration of the PPD and in the reading of results may produce either false-negative or false-positive results. False negatives may also occur due to anergy in immunocompromised individuals with a wide range of concurrent disease conditions. False positives may result from contact with environmental mycobacteria which share common antigens with M. tuberculosis and M. bovis, or from prior vaccination with bacille Calmette Guérin (BCG), a strain of M. bovis which is also antigenically similar to the pathogenic organisms.1,3,4 It is expected that these limitations also apply to use of the TST in non-human primates, including the effects of BCG vaccination which has historically been used occasionally in non-human primates (Andreas Knieriem, personal communication). Furthermore, the efficacy of this test has not been established for the vast majority of non-human primate species, and tuberculin testing practices vary widely between zoos, including differences in the PPD preparation(s) used, antigen strength, and injection site.5
Comparative testing, involving the simultaneous administration of M. tuberculosis or M. bovis PPD and Mycobacterium avium PPD at two different sites, is used commonly in non-human primates to differentiate between TB and infection with environmental mycobacteria. However, interpretation of the results of such tests in humans has shown considerable variability,1 and there have been no evaluations of the reliability of the procedure in non-human primates. The TST also has the disadvantage that it must be read at 72 hours. This introduces the issue of potential non-compliance in human patients4 and compromises the ability to accurately evaluate the result in non-human primates, because frequently the injection site is only observed and not palpated, as it is preferable in most cases to avoid restraining animals again within such a short time period.
Several alternate methods for indirect TB diagnosis have been developed. Lymphocyte transformation assays involve incubation of lymphocytes with mycobacterial antigens. In animals with mycobacterial infection, sensitised T-lymphocytes will undergo cell division in response to the antigens, and the expansion of these cell populations is measured using radiolabelling techniques. This test has been evaluated in deer and found to have 95% sensitivity and 92% specificity; however, its value in many species remains to be demonstrated.5 The major disadvantages of this test are that strict protocols must be followed in the methods and timing of blood collection and laboratory submission, and it is a labour-intensive and lengthy (approximately 7 d) laboratory procedure (Jonathan Streeton, personal communication). It is therefore not very practical or cost effective as a routine screening test.
Enzyme immunoassays (EIA) that detect circulating anti-mycobacterial antibodies have been developed for several animal species. These assays appear to be useful in detecting active TB in several species, but less effective in detecting latent infection.3,5 In cases of active TB, there is a heavy bacterial load and concomitant high levels of circulating antibody resulting from the inability of the immune system to control the infection. However, because mycobacteria are intracellular pathogens which replicate within host macrophages, host defenses are believed to be largely dependent on T-lymphocytes, with antibodies being of only minor importance.3 It is likely, therefore, that only low levels of antibodies are present in cases of latent disease.
The persistence of mycobacteria within macrophages is believed to be due to their ability to switch off the normal process of phagocytosis. The surrounding lymphocytes become cognizant of these persistent bacteria and secrete cytokines, including IFN-γ, in an effort to stimulate the colonized macrophages.6 The CMI, measured by the TST, is dependent on the production of these cytokines by the sensitized lymphocytes at the site of the tuberculin injection, in recognition of the mycobacterial protein.3,6 Based on this phenomenon, Wood et al. developed an in vitro assay for measuring CMI responses. The assay involves overnight incubation of small aliquots of whole blood with tuberculin PPD antigens and mitogen (phytohemagglutinin) to stimulate sensitized lymphocytes to produce IFN-γ. The blood is also cultured with a Nil antigen. The plasma supernatant is then assayed for IFN-γ using an EIA.2,3,7 The Nil antigen control is used to detect IFN-γ in the circulation which, if present, may mask specific responses and make interpretation difficult. The mitogen antigen is used as a positive control to demonstrate that the blood contains immunologically competent T cells capable of producing IFN-γ. Inadequate response to this control may indicate immunosuppression or blood sample deterioration, and the test would be considered invalid.3
This assay was first applied to TB diagnosis in cattle, using bovine and avian PPDs and an EIA specific for bovine IFN-γ (BOVIGAM™, CSL Animal Health, Parkville, VIC, Australia). This test has been extensively trialled and found to have 88–100% sensitivity for culture-confirmed bovine TB (compared with 72% for the skin test) and 94–100% specificity. It has been approved by the USDA and is now used routinely in many countries for TB diagnosis in cattle, buffalo and goats. It is also known to be effective in several exotic hoofstock species.8 The assay was subsequently applied to the diagnosis of TB in humans, using human and avian PPDs and an EIA specific for human IFN-γ (QuantiFERON®-TB, Cellestis Ltd., St. Kilda, VIC, Australia).3,4 This test has been found to have 90% sensitivity and 98% specificity in the diagnosis of latent TB.6 It is now used as a diagnostic test in Australia and was approved by the U.S. FDA in late 2001. Most recently, the assay has been modified for the diagnosis of TB in non-human primates, using bovine and avian PPDs and an EIA specific for primate IFN-γ (PRIMAGAM™, CSL Animal Health, Parkville, VIC, Australia).2 It has been used successfully in gorillas, chimpanzees, orangutans, gibbons, macaques, baboons, mandrills, guenons, vervets, langurs, guerezas, squirrel monkeys, marmosets, tamarins, and lemurs (Stephen Jones, personal communication). It is currently used as a diagnostic test in Australia and, at the time of writing, has approval pending from the USDA.
IFN-γ assays have several advantages over the TST and other indirect diagnostic techniques for TB. The tests require a single patient visit; they are not influenced by tester/observer error; they can be completed in less than 24 hours; the procedure is routine and can be handled by any standard serology laboratory performing EIAs; they do not involve the introduction of foreign protein into an individual and therefore may be repeated as frequently as required; they differentiate between TB and exposure to environmental mycobacteria; and they include assessment of the patient’s current immune status, permitting assessment of the validity of the test response.4,6 The current QuantiFERON®-TB and PRIMAGAM™ tests, however, do not differentiate between IFN-γ responses generated by TB infection and BCG vaccination, hence “false positives” may still occur in BCG vaccinees.1 Recently, specific antigens, ESAT-6 and CFP-10, have been identified which are present in the genome of M. tuberculosis and M. bovis (and three species of atypical mycobacteria rarely associated with disease), but not in BCG or the other non-tuberculosis mycobacteria. Inclusion of ESAT-6 and/or CFP-10 in IFN-γ assays in the future will permit differentiation of these responses.1
The following case investigation demonstrates the use of IFN-γ assays in TB diagnosis. A 20-year-old female western lowland gorilla at Melbourne Zoo was immobilized in January 2002 for investigation of chronic intermittent lameness. Osteoarthritis was diagnosed and routine TB tests performed, including a comparative TST and PRIMAGAM™ assay. These tests both detected a strong response to bovine PPD, indicating infection with M. bovis or M. tuberculosis (Table 1). This was a surprising result, because there has been no known case of TB in any animals at the zoo in the past 30 years. Quarantine of all new arrivals to the zoo, including TB testing of all primates, pre-employment TB testing of all staff with animal contact, and regular surveillance of the TB status of individual primates have all been effective prophylactic measures against this disease entering the collection. TB is not reported in any native or feral animal species found in the zoo grounds, and bovine TB has officially been eradicated from Australia. Our gorilla group lives in a large open-air display, and visitors view the animals either through glass or at a minimum distance of 10 m. TB transmission from visitors, therefore, is not considered a possibility (Jonathan Streeton, personal communication).
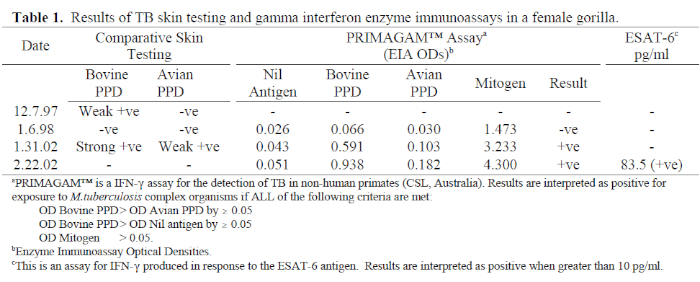
The gorilla was wild caught as an infant in Gambia in 1982. She was subsequently housed with chimpanzees in a rehabilitation-release centre, then relocated to a European zoo in 1990. A single TST was performed on arrival there, with a negative result. She was relocated to Melbourne in Dec 1997; a comparative TST was performed while she was immobilized for placement in the shipment crate. Seventy-two hours later, there was slight induration at the bovine PPD injection site. She was immobilized 3 weeks later for TB investigations including repeat TST, PRIMAGAM™ assay, thoracic radiographs, and specimen collection for acid-fast smears and mycobacterial culture. On this occasion, the TST and PRIMAGAM™ were both negative, and the other diagnostic procedures did not detect evidence of TB. She was therefore released from quarantine.
Following the positive TST in Jan 2002, the animal was immobilized again for TB investigations, including all procedures undertaken in January 1998, with the addition of gastric and colonic endoscopy and biopsy, and PCR on specimens collected for culture. While the PRIMAGAM™ was positive, all other tests were negative for evidence of active disease. Furthermore, it has no clinical signs of active disease. A prophylactic course of isoniazid and rifampicin was implemented.
IFN-γ assays proved very valuable in the investigation of this case:
1. There was a possibility that the TST result in January 1998 was a false negative, either due to errors in injection or reading techniques, or due to immunosuppression, possibly induced by the significant stress of relocation. These possibilities, however, were ruled out as the PRIMAGAM™ result was also negative, and the strong mitogen response indicated immunocompetence. It was concluded from this result that the earlier TST response was therefore likely to be a false positive, possibly due to injection trauma or other trauma to the eyelid induced during the relocation process.
2. It was also possible that the TST result in January 2002 was a false positive, either due to injection technique, exposure to environmental mycobacteria, or previous BCG vaccination. The strong concurrence of the PRIMAGAM™ result with the TST ruled out the first two possibilities. The response to bovine PPD was significantly greater than that to avian PPD, indicating infection with M. bovis or M. tuberculosis. As it was plausible that the gorilla had been given BCG in Gambia, ESAT-6 and CFP-10 were included in the IFN-γ assay in February 2002. These are experimental antigens, provided by the Statens Serum Institute, Denmark, and Cellestis Ltd, Australia. Both produced strongly positive results which clearly ruled out the possibility of a BCG reaction because these antigens are not present in the BCG genome. Only the result for the ESAT-6 assay appears in Table 1 because there was insufficient blood to quantify the CFP-10 result. These results confirmed that she is infected with M. bovis or M. tuberculosis. There is no indirect diagnostic test that can differentiate between these two organisms.
3. The source of the gorilla’s infection has not been identified; however, PRIMAGAM™ results from January 1998 are worth some attention. The enzyme immunoassay optical density (OD) bovine PPD was greater than OD avian PPD by 0.036, and OD bovine PPD was greater than OD Nil antigen by 0.040. While both differences are less than the test cut-off point of 0.05, they are greater than those in all other adult gorillas tested (see Table 2), and these values may in fact be reflective of recent infection with TB. The protocol for screening humans following TB exposure is to perform two TSTs 3 months apart, because CMI does not develop immediately following infection, and a case of early disease may be missed if the patient is only tested once.6 Australian quarantine protocols required the animal to be held in post-arrival isolation for 60 days, and a single TST performed in that time. The outcome of this case suggests that a longer post-arrival quarantine period is appropriate for imported primates, including two TSTs 3 months apart.
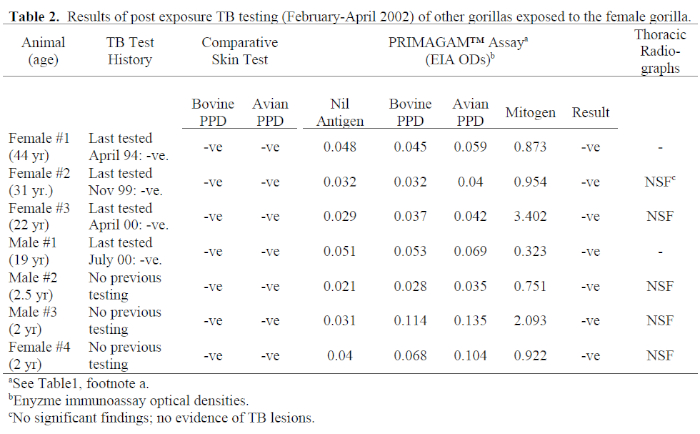
4. Subsequent to the diagnosis of TB in this animal, all other gorillas in the group and all personnel who had worked closely with her or her wastes were tested for TB using both TST and IFN-γ tests. Results of these tests are presented in Tables 2 and 3. All gorillas were negative for TB on both tests, but the IFN-γ results of male #3 and female #4 included a mild response to avian PPD which exceeded the bovine PPD response, indicating exposure to environmental mycobacteria. These are hand-reared infants which share an enclosure not accessed by other group members. The strong mitogen response reported for all gorillas ruled out the possibility of false negatives due to anergy. This is significant, because all animals except females #1 and #3 were infected with varicella-zoster virus either at or close to the time of testing. If TSTs alone had been performed, immunosuppression by concurrent disease would have been considered a possibility.
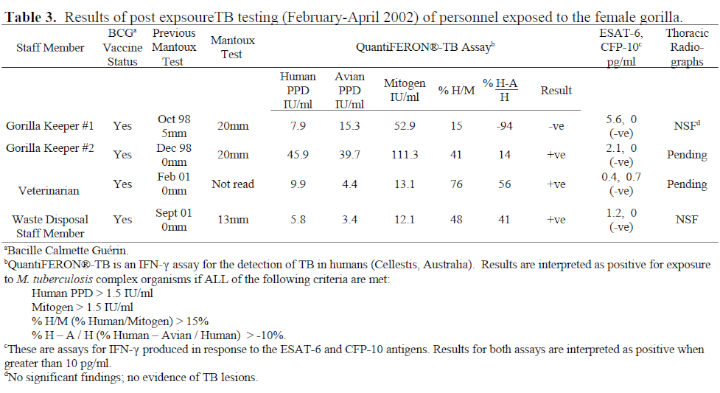
5. Nineteen staff members were tested, all of whom had been Mantoux tested 1–4 years earlier. Three people had significant conversions in their Mantoux responses. One other person (veterinarian) did not present for Mantoux test reading but had a positive QuantiFERON®-TB result. All four had histories of BCG vaccination, but as they had shown no or negligible response to their previous Mantoux, these recent reactions caused some concern. The IFN-γ assays proved very useful in the interpretation of these responses (see Table 3). The QuantiFERON®-TB results of gorilla keeper #1 showed a dominant response to avian PPD, indicating that her TST conversion was due to exposure to environmental mycobacteria. The IFN-γ assays of the other three were all positive for M. tuberculosis complex organisms; however, their ESAT-6 and CFP-10 assays were negative, clearly indicating that their responses were to BCG, and they are not infected with M. bovis or M. tuberculosis. If IFN-γ assays had not been available, gorilla keepers #1 and #2 would have been diagnosed as having latent TB on the basis of existing criteria (Mantoux induration >15 mm in BCG vaccinees) and prescribed a 9-month course of isoniazid. All other tested staff members showed no recent Mantoux conversions or significant IFN-γ results.
The use of IFN-γ assays in this investigation has permitted us to conclude at this stage that there is no evidence of disease spread from the infected gorilla. All contact gorillas and staff will be retested 3 months after their first test. Meanwhile, strict quarantine procedures are in place within the zoo.
Acknowledgments
The author would like to thank Dr. Stephen Jones of CSL Animal Health for performing the PRIMAGAM™, ESAT-6 and CFP-10 assays, and for his invaluable advice in the investigation of this case. Sincere thanks are also due to Dr. Jonathan Streeton for his generous advice and support in the investigation and management of the gorilla and contact personnel. Thank you also to Dr. Peter Anderson, Statens Serum Institute (Copenhagen, Denmark) and Dr. Jim Rothel, Cellestis Ltd. (Melbourne, Australia) for providing the experimental ESAT-6 and CFP-10 antigens, and to the staff of Melbourne Zoo for their professionalism and cheerful cooperation throughout the investigation.
Literature Cited
1. Anderson, P., M.E. Munk, J.M. Pollock, and T.M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet. 356: 1099–1104.
2. C.S.L. Veterinary Division. 1998. PRIMAGAM™: Non-human primate gamma interferon test: an assay of cell mediated immunity and detection of tuberculosis infection in non-human primates. Manufacturer’s instructions for use. C.S.L. Limited, Melbourne, Australia.
3. Desem, N., and S.L. Jones. 1998. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin. Diagn. Lab. Immunol. 5(4): 531–536.
4. Mazurek, G.H., P.A. LoBue, C.L. Daley, J. Bernardo, A.A. Lardizabal, W.R. Bishai, M.F. Iademarco, and J.S. Rothel. 2001. Comparison of a whole blood interferon γ assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. J. Am. Med. Assoc. 286(14): 1740–1747.
5. Mikota, S.K., and J. Maslow. 1997. Theoretical and technical aspects of diagnostic techniques for mammalian tuberculosis. Proc. Am. Assoc. Zoo Vet. Pp. 162–165.
6. Streeton, J.A., N. Desem, and S.L. Jones. 1998. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int. J. Tuberc. Lung Dis. 2(6): 443–450.
7. Wood, P.R., L.A. Corner, and P. Plackett. 1990. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of γ interferon. Res. Vet. Sci. 49: 46–49.
8. Wood, P.R., and S.L. Jones. 2001. BOVIGAM™: an in vitro diagnostic test for bovine tuberculosis. Tuberculosis. 81(1–2): 147–155.