|
|
|
|
Chemotherapy: Safety and Use
Rodney L. Page MS, DVM: Diplomate ACVIM (Internal Medicine/Oncology)
College of Veterinary Medicine, Cornell University
General Considerations
Chemotherapeutic agents can be distinguished from all other pharmacologic agents by the fact that while other agents act to modify a cellular or tissue reaction, the desired endpoint for classic chemotherapeutic agents is cell death. The ability to achieve any therapeutic benefit relies on the differential sensitivity of the drug between normal tissue and tumor. Basic differences between a tumor cell population and normal cells exist in the ability of tumor cells to proliferate unrestricted given the correct environment and in their capacity to repair DNA damage. However, the growth characteristics of tumors vary considerably depending on the microenvironmental conditions and the extent of the malignancy (degree of undifferentiation). This implies that some tumors are not appreciably different from rapidly proliferating normal tissue such as bone marrow progenitor cells, intestinal epithelial lining and germ cells.
Since a narrow therapeutic index exists for antineoplastic agents it is essential that dose prescription be precise. Unfortunately, no ideal dose prescription base currently exists. Body surface area (BSA) estimates metabolic rate and may therefore, predict toxicity more accurately than body weight if the drugs' action or metabolism is related to some metabolic process. A disproportionately greater dose is given to small dogs if dose is based on BSA on the assumption that meabolic rate is increased and thus drug biotransformation and elimination is also increased. However, recent evidence suggests that, for certain drugs, BSA fails as a uniform prescription base and can result in a relative overdose in small dogs. The problem with BSA calculation in dogs is that the information used to estimate the BSA is based on outdated and insufficient information. In addition, breed conformation differences are not accounted for so that a 20 pound Dachshund has the same BSA as a 20 pound Whippet. Finally, the majority of chemotherapeutic agents affect bone marrow function which may not be accurately estimated by a measure of metabolic rate.
The route of drug administration and the administration regimen will obviously determine the drug exposure to the central compartment. Bioavailability of orally administered compounds may be a significant variable which alters the expression of toxicity and effficacy. For drugs administered intravenously the type of infusion given will have a significant impact on the serum drug exposure. These considerations should be examined in preliminary pharmacologic studies so that by the time a drug is recommended for more extensive therapeutic investigation the best means of administering the compound will be tested. Novel means of drug administration have certain pharmacologic benefits. Intralesional, intra-arterial or intracavitary chemotherapy significantly increases the local drug exposure wihout increasing systemic drug levels. The therapeutic benefit of these regimens is yet to be fully investigated.
The initial dose of chemotherapy is administered based on the best estimate of the maximally tolerated dose as defined above. It is clear that numerous factors impact the manifestation of treatment in each individual. Therefore, dose adjustments following the initial dose may be necessary.
|
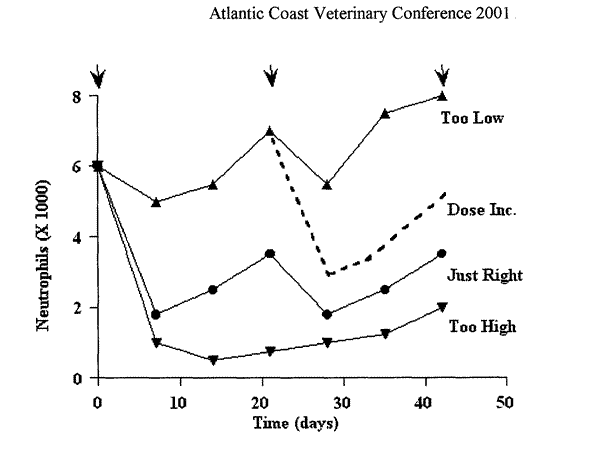
The goal of therapeutic drug monitoring is to adjust the dose of a chemotherapeutic agent based on subclinical and reversible evidence of toxicity. Most chemotherapeutic agents affect the neutrophilic leucocyte, therefore following the effect of treatment on the circulating neutrophil count during the first 1 or 2 treatment courses will insure optimum doses for that patient. The figure illustrates 3 different scenarios following treatment with the same dose of drug in different patients. The arrows indicate the days of treatment. The dotted line indicates the data following an increase in drug dose at the second treatment date for the dose that is considered too low.
Strategies for Application of Chemotherapy
Combination chemotherapy:
Agents can be combined if they are effective as single agents against the tumor being treated and if there is non-overlapping toxicity. This effectively increases the potential impact on tumor damage without increased normal tissue damage. Multiple phases of the cell cycle can be attacked which may also help eliminate tumor population subsets exhibiting differing resistance characteristics.
Treatment strategies:
1) Cyclic, combination therapy - multiple agents used in a repetitive cycle for a given period of time.
2) Induction therapy- an intensive period of treatment to produce the greatest response.
3) Maintainence therapy- a different group of drugs used to maintain the response once a remission has been achieved.
4) Palliative therapy - Treatment to ameliorate the clinical signs of cancer without the intent of producing a cure.
5) Adjuvant chemotherapy - Use of antineoplastic agents following primary therapy of the tumor with another treatment modality (surgery, radiation) in a preplanned attempt to eliminate residual, often microscopic tumor.
Clinical Safety With Chemotherapy: Important Considerations
Chemotherapeutic drugs have the potential to be mutagenic, embyotoxic, teratogenic, carcinogenic, and cytotoxic. They may be irritating to the skin, and can enter the body by many ways; iatrogenically; by absorption through mucous membranes, the eyes (including soft contact lenses), and the skin, or by inhalation. Below are a few simple things you can do to prevent accidental exposure.
If You Are Pregnant, Breast Feeding Or Trying To Conceive: You should avoid all contact with these drugs. Chemotherapy targets are rapidly dividing cells, and can be very harmful to your child. Avoid contact with the animal and its wastes for at least 72 hours after their last treatment with chemotherapy.
Handling The Medicine: All chemotherapeutic drugs should be prepared in a designated area. This space needs to be of low traffic, well ventilated, and close to an eyewash. Ceiling fans need to be turned off. It is recommended that preparation be done under a vertical airflow fume hood. Use of a horizontal airflow fume hood is not recommended, as these may blow the aerosolized drug into the face of the preparer. A puncture-proof container marked for chemotherapeutic waste only has to be accessible. Refrain from smoking, applying makeup, adjusting contact lenses, eating, and chewing gum while handling these drugs. If such facilities are not practical, arrangements can be made with a local pharmacy or hospital for the preparation of the medicine.
You should wear disposable un-powdered latex gloves (not vinyl). Do not use polyvinyl chloride (PVC) gloves, as these are permeable to some of the therapeutic agents. Goggles and an aerosol mask or a plastic face shield are also recommended. Wash your hands thoroughly after contacting the drugs.
Preparation Of Injectables: It is best to arrange the needed equipment prior to opening the drug vials.
Syringes fitted with luer-locks are recommended to reduce the chance of aerosolization. Choose syringes that will not need to be filled above three-fourths of their capacity. Labels for the syringes and clear resealable transport bags (Ziploc™) should be prepared beforehand. Take the time to have the reconstitution fluids and flushing solutions ready, the intravenous lines primed and attached, and the needles attached to the syringes. Place a disposable plastic lined towel over the counter area. Use an alcohol soaked (70%) gauze to wipe the exposed vial tops.
Many anticancer agents are now supplied in solutions such that reconstitution of powdered drug is unnecessary. Adding diluent to powdered drug should be accomplished without spilling or aerosolizing any product. Use care to neutralize pressure in vials. Wrapping the vial and syringe in gauze pads during injection and withdrawal of fluid is recommended to absorb any product spilled during reconstitution.
Any items, which may have come into contact with the drug, should be swabbed with an alcohol soaked gauze. All waste, which may have contacted the agent, must be disposed of in the hazardous waste container. Do not recap or clip needles, but do place them into a sharps container that is dedicated for chemotherapeutic waste only. The gown and outer pair of gloves can now be removed and disposed of. Next remove the goggles and the other pair of gloves. Wash your hands thoroughly with soap and water.
Preparation Of Oral Drugs: Wearing gloves is recommended but use of a fume hood is not required.
Wash the counter surface with detergent and water after counting the pills. It is safer to round down the drug dose to one closest to the tablet size available. This prevents aerosolization that occurs from breaking, crushing or cutting the pills.
Patient Housing: A hazardous drug card, which lists the date of treatment and drug given, will need to be placed on the animal's cage. The cage should be lined with disposable paper bedding. Soiled bedding is considered to be hazardous waste. Soap and water is recommended for cleaning the cage. Latex gloves should be worn when handling the soiled bedding.
Cleaning Up After The Patient: It is normal for the whole drug or metabolites of the medicine the patient is taking to end up in their urine and feces. It is a good idea to walk the animal away from public areas for at least 24 hours after they have been treated. Walking the pet often will reduce the likelihood they will make a mess in the cage. Gloves need to be worn when picking up the animal's droppings. If possible, hose the area in which they urinated. Put any feces or vomit into a plastic bag and then place them into the hazardous waste container or into a toilet. If a toilet is used, drop the feces/vomit close to the water to avoid splashing, and flush. Gloves, plastic bags and cat litter all should be placed into the biohazard bag. This bag should be stored away from high traffic areas.
Disposal: All chemotherapeutic drug waste should be incinerated at 1,000o C (1,800o F) by an Environmental Protection Agency (EPA) approved facility. Often arrangements can be made with a local human hospital for proper disposal.
Safe Administration of Chemotherapy:
Only experienced and well trained individuals should administer chemotherapy to patients. Many compounds must be administered intravenously through an indwelling catheter that was placed without any complications. Several agents are extremely caustic if administered extravenously. Therefore, close observation of the patient during injection or infusion is necessary. Any concern about the placement of the catheter or the patency of the catheter during treatment should result in interruption of treatment to insure that the catheter is still patent. Don't take chances with this. If necessary sedate the patient for catheter placement and infusion. This is not generally recommended.
Managing Common side-effects of Chemotherapy: Client Instructions
Vomiting
Withhold food and water for 12 hours, and then offer small amounts of water. If your pet does not vomit after drinking the water, offer small amounts of bland foods such as boiled chicken or boiled hamburger with boiled white rice. If still no vomiting, gradually reintroduce the pet's normal diet in about three days. Call the hospital if the vomiting is severe or is accompanied by a fever greater than 103oF or persists longer than 24 hours.
Diarrhea
Offer your pet bland, easily digestible foods such as cottage cheese, boiled chicken or hamburger and white rice. Gradually reintroduce your pet's normal diet.
Pepto-Bismol can be given at one tablespoon per 15 pounds of body weight (dog) three times a day (every 8 hours) or 1/2 a tablet per every 7 pounds of body weight two times a day (every 12 hours). Call the hospital if the diarrhea persists for more than 48 hours or if it is associated with a fever greater than 103oF.
Dehydration
Dehydration can develop following vomiting, diarrhea, excessive urination or fever and may result in a prolonged recovery. Your dogs gums should be moist and the skin should feel soft and compliant. If your pet is not vomiting, fresh water should always be available. Call the hospital if gums are persistently dry or if the skin does not feel normally supple. Fluid administration may be necessary to speed recovery.
Low White Blood Cell Count
The white blood cell count is expected to drop below normal after treatment, but will return to normal bythe next treatment. This should not cause a problem unless the white blood cell count drops too low. When the white blood cell count drops too low, the body has difficulty fighting off infections. Infections may occur between 7-21 days after the drug is given. If this happens, symptoms may include a fever (temperature >103oF), lethargy (tiredness), vomiting, diarrhea, and a poor appetite. A blood sample will be evaluated at the 7 day time point. If the blood count is low we may dispense antibiotics to prevent an infection.
If your pet shows any of the symptoms mentioned above, take your pet's temperature if you can (normal temperature is 100-102.5oF). If the temperature is greater than 103oF or if you cannot take the temperature, you should call the hospital immediately. Your pet may need to be admitted to the hospital.
|
|

Copyright ACVC