Knowledge Is Power: Effective Anesthetic Monitoring and Logical Crisis Management
Sathya K. Chinnadurai, DVM, MS, DACZM, DACVAA
Abstract
This lecture serves as a refresher on the effective use of common anesthetic monitors, including the challenges of using those monitors in non-domestic species. We will discuss how the machines work, the relevant physiology and nuances with some zoo species. In addition to a review of the function of monitors, we will discuss a systematic approach to the diagnosis and treatment of common anesthetic problems. We will focus on respiratory derangements such as hypoxemia and hypoventilation and briefly discuss hypotension.
Introduction
In veterinary school, students are taught to identify a problem, generate a list of differentials and systematically rule in or rule out those differentials, until we come to a diagnosis. Unfortunately, when it comes to anesthetic management, and especially anesthetic emergency management, we often fail to take the same linear and logical approach.
The first step in diagnosing an anesthetic problem is determining if you have a machine problem or a patient problem. We do this by instinct sometimes, but too often, we write off a real problem as an equipment problem. The easiest way to distinguish patient problems from equipment problems is to employ redundant monitors. If the patient is bradycardic on the ECG, check the pulse oximeter or auscultate with a stethoscope. Sometimes the interpretation of the redundant monitor is less straightforward. If the patient’s blood pressure drops suddenly, check the heart rate. A normal animal should compensate for a sudden drop in blood pressure with a compensatory increase in heart rate. Very rarely does an acute change in heart rate, respiratory rate, blood pressure, oxygen saturation or expired CO2 occur without a deviation in another parameter.
Before we start talking about treating anesthetic problems, we need to spend time talking about anesthetic monitors. Without a solid understanding of how the monitors work, we cannot know how accurate the information we are relying on is. The pulse oximeter, capnograph and oscillometric blood pressure monitor are the commonly used “standard anesthetic monitors.” It is important to remember that even though these devices are widely used in zoo medicine, very few have been objectively evaluated in a non-domestic species.
Anesthetic Monitor Review
The Pulse Oximeter
The pulse oximeter uses photoplethysmography to determine arterial hemoglobin saturation. It uses light-emitting diodes and photosensors to detect pulsatile blood flow and determine a ratio of oxygenated to deoxygenated hemoglobin based on differential light absorption. Accurate performance depends on multiple factors including good tissue perfusion, good pulse quality, thin epidermis, hemoglobin and oxyhemoglobin that absorb light at the required frequencies.1 Species difference in hemoglobin structure could affect the accuracy of the reading. In cases of anemia, a patient may have excellent hemoglobin saturation, but an overall deficiency in hemoglobin will still result in poor oxygen delivery. Other sources of error include patient movement, bright ambient light, and the fact that most devices are not calibrated to read accurately below 80%.
The Capnograph
Capnography is arguably the most useful anesthetic monitor and often the most underused. Effective capnography can identify conditions which might lead to hypoxia before a patient is hypoxic. It is one of the most reliable ways of detecting cardiac arrest and airway obstruction. The capnograph can also be used for confirming endotracheal tube placement in challenging intubations, detecting disconnections and leaks and problems with soda lime and one-way valves.
The capnograph typically uses infrared spectrometry to determine partial pressure of CO2 in expired gas.1 This generates three pieces of information 1) end-tidal CO2 partial pressure, 2) a capnograph waveform and 3) a respiratory rate. In order for CO2 to be detected and read out on a capnograph, 3 steps have to take place:
1. Cellular metabolism has to produce CO2
2. Circulation has to bring CO2 from the periphery to the lungs
3. The lungs have to be ventilating for the CO2 to be exhaled and detected by the monitor.
Abnormalities in end-tidal CO2 can occur due to derangements in any of those 3 parameters. In a healthy “normal” animal, end-tidal CO2 provides a close approximation of arterial partial pressure of CO2, the indicator of ventilatory function and respiratory pH balance. Hypoventilation can cause increases in ETCO2 and PaCO2. Hyperventilation can cause decreases in both. There are also numerous scenarios when the discrepancy between expired and arterial CO2 may grow. These include any state of poor perfusion, which decreases cardiac output and pulmonary perfusion. With less blood delivered to the lungs, there is less CO2 exhaled, but there is no change in CO2 production.
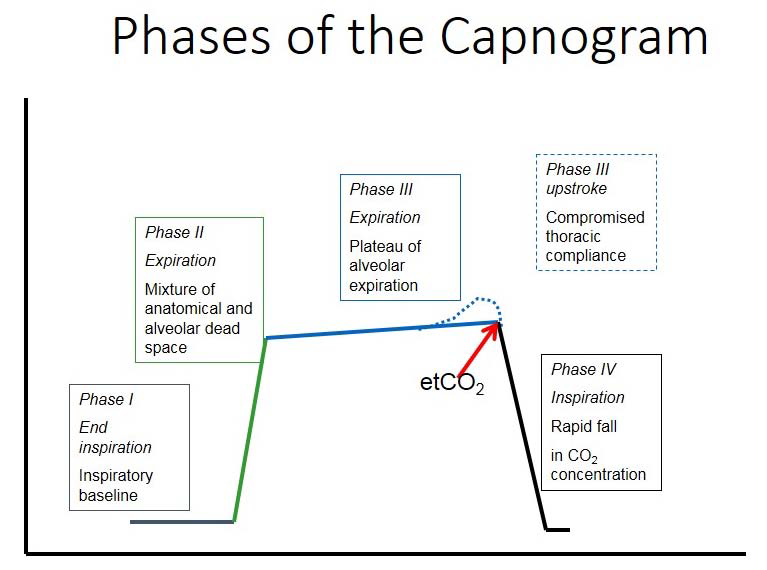
Many practitioners use their capnograph simply for one value: end-tidal CO2. That single number can be used as an effective indicator of ventilatory function, but the capnograph waveform provides many more valuable pieces of information about equipment and animal function. It can track changes in not only ventilation, but cardiac output and metabolic rate. The four phases of the capnogram that should be interpreted are pictured below.
Phases of the Capnogram
The Oscillometric Blood Pressure Monitor
In many cases, blood pressure itself is not a primary concern. Blood pressure is an easily measured surrogate for cardiac output and perfusion. Blood pressure is composed of two things: cardiac output and systemic vascular resistance (SVR). Cardiac output is made of two things: heart rate (HR) and stroke volume (SV). Most derangements of blood pressure involve a change in HR, SV or SVR.
The device inflates an air-filled cuff to occlude blood flow in an artery. It then decreases pressure in the cuff in a stepwise fashion to detect changes in the frequency of oscillation produced by pulses in blood vessels. If the time between successive pulses is sufficiently long, the device may provide an inaccurate reading.1 This source of error is commonly seen when oscillometric devices are used on animals with low heart rates, such as reptiles or equids. Other sources for failure of the oscillometric device include movement by the patient and poor signal quality. Hypotensive patients with poor peripheral perfusion often generate errors in blood pressure measurement. Unfortunately, these are often the patients that need accurate readings the most.
Systematic Anesthetic Troubleshooting
Years of clinical practice often result in some instinctual responses to anesthetic problems. The patient is tachycardic, it must be light, and the anesthetist should turn up the gas. This approach is very piecemeal and often ends up in misdiagnosis and mistreatment. We will look at some of the most common anesthetic complications that we deal with every day, but we will look at them with a problem-based approach. With each problem “symptom” we will list 3–4 differentials.
Hypotension
Most causes of hypotension involve a change in HR, SV or SVR. Changes in the different parameters are interrelated. Aside from being important for basic physiology, remembering these three components gives you a set list of differentials for why your patient is hypotensive.
1. Decreased HR: opioids, high vagal tone, α2 agonists
2. Decreased SVR: vasodilation: gas anesthesia, acepromazine, sepsis
3. Decreased SV: decreased preload/venous return: dehydration, abdominal mass, positive pressure ventilation
Steps in treating hypotension:
1. Assess and adjust anesthetic depth. Too often, a vaporizer is set at a given percent and forgotten for the remainder of the procedure. Inhalant gases affect vascular tone and contractility and are potent hypotensive agents. Use only as much as you need.
2. Fix preload. This can involve fluids such as crystalloids and colloids. This could also be due to poor venous return. Animals with abdominal masses or large fetuses have decreased return of venous blood through the caudal vena cava to the heart, especially in dorsal recumbency and may benefit from being rolled into lateral recumbency. Additionally, positive pressure ventilation also decreases venous return and may need to be adjusted in the face of hypotension.
3. Fix vascular tone. Adjusting depth may increase sympathetic stimulation and promote vasoconstriction. Drugs such as ephedrine, phenylephrine stimulate α1 receptors and cause vasoconstriction.
4. Fix cardiac output:
- If animal is bradycardic, consider atropine or glycopyrrolate
- Increase contractile force of the heart by using pressors such as dopamine or dobutamine
Tachycardia and Bradycardia
Both are integrally tied to the changes in blood pressure. In most situations the absolute heart rate is not nearly as important as the role heart rate plays in cardiac output and perfusion. Instinctually people want to treat brady- and tachycardia, especially since we often are just measuring heart rate and respiratory rate. It is crucial to interpret heart rate in relation to blood pressure and cardiac output. It is rarely necessary to treat bradycardia in a normotensive, nonarrhythmic patient. For abnormalities in heart rate, treating the primary problem is the primary concern.
Reasons for tachycardia:
1. Compensation for low blood pressure (baroreceptor response)
2. Compensation for hypoxemia (chemoreceptor response)
3. Sympathetic stimulation from noxious stimuli (i.e., patient is too light)
Reasons for bradycardia:
1. Response to high blood pressure (baroreceptor response)
2. Direct vagal stimulation from anesthetic drugs (opioids, α2 agonists)
3. Excessive anesthetic depth
Hypoxia
In terrestrial mammals, hypoxemia is defined as an arterial partial pressure of oxygen less than 60 mm Hg or an SpO2 of less than 90%. Hypoxia occurs for one of 5 reasons:
1. True hypoventilation. Either the patient is not breathing enough or it has an obstruction, keeping it from breathing. In these cases, the patient has a high PaCO2 and a low PaO2.
2. Ventilation/perfusion mismatch:
a. Atelectasis
b. Dead-space ventilation
3. Anatomic right-left shunt. Uncommon, unless you are a reptile and are built for this.
4. Low inspired FiO2 (the animal is breathing a low oxygen mixture). This is rare under anesthesia.
5. Diffusion impairment. Very rare.
Of the above choices, 1 and 2 are, by far, the most common. Hypoventilation can be ruled in or out only with a capnograph or blood gas to determine CO2. If the ETCO2 is high and the SpO2 is low, breathe more frequently. If the ETCO2 is low or normal and the SpO2 is low, there is most likely a ventilation-perfusion mismatch, such as atelectasis. In this case fewer, deeper, larger breaths will help but only increasing rate will not.
Tachypnea
Again, we often assume that if a patient’s respiratory rate increases, that it is light. In truth, there are likely 4 reasons why a patient is tachypneic under anesthesia:
1. It is hypercapnic
2. It is hypoxemic
3. It is light and responding to noxious stimulus
4. It is hot and trying to lose heat
Either CO2 is too high, oxygen is too low, the patient is light/stressed/painful (i.e., inadequately anesthetized) or it is too hot. Instinctually turning up the gas only addresses one of these options. When noticing any change in respiratory rate and character, do a spot check. What is the ETCO2, the SpO2, body temperature and depth of anesthesia? If something does not make sense, run a blood gas and make sure your equipment is working.
Literature Cited
1. Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2008.