Abstract
The harvesting of liver samples for toxicologic and other laboratory analyses is frequently undertaken in free-ranging fish in order to evaluate accumulations of various pollutants and chemicals.1-13 However, commonly used techniques of collecting liver are lethal and unacceptable when dealing with charismatic, threatened or endangered species. We report the use of a non-lethal, single-entry, endoscopic technique using saline infusion to examine and collect large liver samples using optical biopsy forceps (62046GS, Karl Storz Veterinary Endoscopy America Inc [KSVEA], Goleta, CA USA; Figure 1) from 15 free-ranging shovelnose sturgeon (Scaphirhynchus platorynchus), and one pallid sturgeon (S. albus). Under tricaine methanesulfonate general anesthesia, a 1–2 cm ventral midline skin incision permitted the introduction of the forceps, which incorporated a 5 mm telescope (62033APA, KSVEA). Liver examination and liver biopsies up to 1.4 grams in weight, and representing up to 14% of total liver tissue were successfully obtained. All fish made uneventful recoveries and those that were subjected to necropsy examinations the following day failed to indicate any significant hemorrhage or iatrogenic trauma. The use of large optical biopsy forceps are recommended as a practical, non-lethal alternative for the collection of large liver biopsies from sturgeon and other fish.
Figure 1. Endoscopic optical biopsy forceps
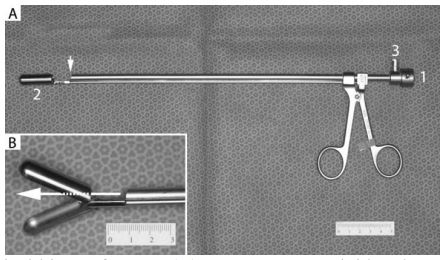
(A) 5 mm x 29 cm rigid endoscope is inserted through the opening (1) such that the terminal lens is positioned as shown by the arrow. This provides a clear view of the large biopsy cups (2). The port (3) provides an ingress for sterile saline to create the necessary insufflation. (B) Close-up of the large biopsy forceps in the open position. Holes at the back of the biopsy cups permit direct observation of the tissue to be sampled (arrow).
Acknowledgments
This project was funded by the Dredging Operations and Environmental Research program. The authors are grateful to Dr Christopher Chamness and Karl Storz Endoscopy for building the prototype optical biopsy forceps that is now available commercially, and supporting endoscopy research and development at the University of Georgia’s College of Veterinary Medicine. Assistance in the field was provided by Jay Collins, Neil Douglas, Kathie Eagles, Nick Friedenberg, Audrey Harrison, Phil Kirk, William Bradley Lewis, Thomas Parker, and Todd Slack. Permission to collect pallid sturgeon was granted by the US Fish and Wildlife Service.
Literature Cited
1. Almar, M., L. Otero, C. Santos, and J. Gonzalez Gallego. 1998. Liver glutathione content and glutathione-dependent enzymes of two species of freshwater fish as bioindicators of chemical pollution. J. Environ. Sci. Health, Part B 33:769–783.
2. Blanch, G.P., A. Glausch, V. Schurig, R. Serrano, and M.J. Gonzalez. 2005. Quantification and determination of enantiomeric ratios of chiral PCB 95, PCB 132, and PCB 149 in shark liver samples (C. coelolepis) from the Atlantic Ocean. J. High Resolut. Chromatogr. 19:392–396.
3. Blumer, M. 1967. Hydrocarbons in digestive tract and liver of a basking shark. Science. 156:390–391.
4. Broeg, K., W. Kaiser, S. Bahns, and A. Koehler. 2008. The liver of wrasse - morphology and function as a mirror of point source chemical impact. Mar. Environ. Res. 66:191–192.
5. Chuiko, G.M., D.E. Tillitt, J.L. Zajicek, B.A. Flerov, V.M. Stepanova, Y.Y. Zhelnin, and V.A. Podgornaya. 2007. Chemical contamination of the Rybinsk Reservoir, northwest Russia: relationship between liver polychlorinated biphenyls (PCB) content and health indicators in bream (Abramis brama). Chemosphere 67:527–536.
6. Fent, K., and J. Hunn. 1996. Cytotoxicity of organic environmental chemicals to fish liver cells (PLHC-1). Mar. Environ. Res. 42:377–382.
7. Hagenaars, A., D. Knapen, I.J. Meyer, K. van der Ven, P. Hoff, and W. De Coen. 2008. Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio). Aquat. Toxicol. 88:155-163.
8. Hartley, W.R., A. Thiyagarajah, and A.M. Treinies. 1996. Liver lesions in the gar fish (Lepisosteidae) as biomarkers of exposure. Mar. Environ. Res. 42:217–221.
9. Serrano, R., M. Fernández, R. Rabanal, M. Hernández, and M.J. Gonzalez. 2000. Congener-specific determination of polychlorinated biphenyls in shark and grouper livers from the Northwest African Atlantic Ocean. Arch. Environ. Contam. Toxicol. 38:217–224.
10. Serrano, R., M.A. Fernández, L.M. Hernández, M. Hernández, P. Pascual, R.M. Rabanal, and M.J. González. 1997. Coplanar polychlorinated biphenyl congeners in shark livers from the North-Western African Atlantic Ocean. Bull. Environ. Contam. Toxicol. 58:150–157.
11. Stehr, C.M., M.S. Myers, L.L. Johnson, S. Spencer, and J.E. Stein. 2004. Toxicopathic liver lesions in English sole and chemical contaminant exposure in Vancouver Harbour, Canada. Mar. Environ. Res. 57: 55–74.
12. Storelli, M.M., G. Barone, A. Storelli, and G.O. Marcotrigiano. 2011. Levels and congener profiles of PCBs and PCDD/Fs in blue shark (Prionace glauca) liver from the South-Eastern Mediterranean Sea (Italy). Chemosphere 82:37–42.
13. Storelli, M.M., A. Storelli, and G.O. Marcotrigiano. 2005. Concentrations and hazard assessment of polychlorinated biphenyls and organochlorine pesticides in shark liver from the Mediterranean Sea. Mar. Pollut. Bull. 50 850–855.