Introduction
Acyclovir is a synthetic purine nucleoside analogue (guanosine) with in vivo and in vitro antiviral activity against varicella zoster and herpes simplex types 1 and 2 in humans.4,7 Inhibitory activity is due to incorporation, inhibition and inactivation of herpesviral DNA.4,7 Acyclovir is marketed as an oral preparation and appears to have the least potential for adverse effects when compared to other antiviral drugs.4 Previous studies of acyclovir in quaker parakeets reported efficacy and few adverse effects at 40 mg/kg IM and 80 mg/kg PO TID as initial trial dosages.5,6
Acyclovir was used in tragopans (Tragopan caboti and T. temminckii) to determine absorption, distribution, and linearity of the drug after a single dose. This study was designed in response to multiple peracute deaths in multiple pheasant species at the Bronx Zoo. Affected birds showed minimal or no clinical signs prior to death. The diagnosis of herpesviral infections on postmortem histopathologic evaluation encouraged the need for investigation of a potential treatment for future cases. There is no known published information on the efficacy or safety of acyclovir in tragopans or on herpesvirus infections in pheasant species.
Methods and Materials
Five healthy adult tragopans, three males and two females weighing 0.96 to 1.8 kg, at the Wildlife Conservation Society (Bronx, NY) were in the study. Birds were fasted 12 hours prior to drug administration, and no treatments were given for at least 1 month prior to study initiation. Each bird was given doses of 40, 80 and 120 mg/kg oral suspension (Alpharma USPD Inc., Baltimore, MD, USA) by gavage. Blood samples of approximately 1 ml per sample were obtained from the jugular, deep ulnar, brachial or metatarsal veins at 1, 2, 6, 12 and 24 hours post-drug administration. Trials were repeated at 1- to 2-week intervals to allow for drug elimination from the subjects.
Results were obtained with reverse phase HPLC with a C-8 column and UV detection.1,2,10 Validation was done with banked, untreated plasma from the same birds used in the study.
Results
All animals were clinically healthy throughout all treatments as determined by physical examination, hematology and plasma biochemistries. Complete blood counts preformed prior to and during the study revealed no evidence of abnormalities that would indicate clinical illness.
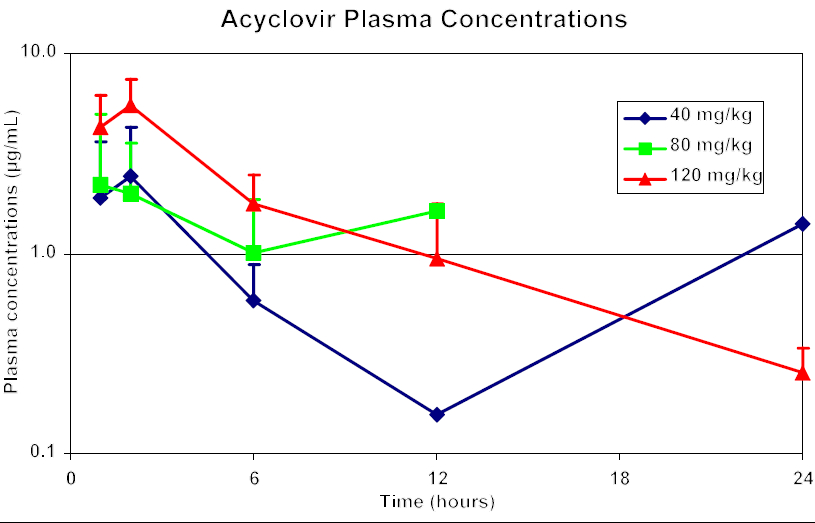
The plasma acyclovir concentration vs. time curve is shown in Figure 1. The AUCtlast significantly increased following the 120 mg/kg dose when compared to both of the lower doses, but not from 40 to 80 mg/kg. This increase was not linear. The values for Cmax also showed similar results, but the increase was only significant between the 40 and 120 mg/kg treatments. There were no detectable levels of acyclovir in plasma at 24 hours with 80 mg/kg dosage.
Figure 1. Plasma concentration of acyclovir versus time
Discussion
Acyclovir is probably the most readily available, effective compound to administer for treatment of herpes virus in captive avian species, at a reasonable cost. Potential for side effects also appears to be less with acyclovir (in comparison to the basic antiviral chemical compounds famciclovir, valacyclovir and interferon).4,7-9 Cost and decreased potential for side effects make acyclovir appealing to the clinician with potential herpesviral infection in their avian collection. These facts also make acyclovir attractive for consideration for future viral studies in birds.
The nonlinearity of acyclovir pharmacokinetics in tragopans makes dose extrapolation difficult, if not dangerous. When pharmacokinetic parameters do not respond in a linear fashion (i.e., as dose doubles, plasma Cmax and AUC double), then one cannot reliably predict the pharmacodynamic effects or efficacy of a given drug. However, the results from this study suggest that 120 mg/kg PO BID may be the best dosage in this species to achieve effective plasma concentrations for treatment and prevention of herpesvirus infections. Precaution should be taken with prolonged therapy, as long-term chronic toxicity has not been evaluated in this species.
A detectable plasma level of acyclovir was noted at 24 hours with 40 mg/kg dosage only in one bird, which may be attributed to individual variation in metabolism or absorption of the drug.
A moderate elevation in bile acids was noted on several birds during the study. Birds were not fasted prior to phlebotomy for biochemistry samples. Increased bile acids in these birds could be due to postprandial sampling or liver compromise. Lack of adequate bile acid reference values for this species prohibits definitive interpretation of these results.
Acknowledgments
Special thanks to Lisa B.E. McCarthy for her diligent assistance in sample collection. Also, thanks to Susan Cardillo and the veterinary technicians at WCS for their assistance in collecting and processing samples.
Literature Cited
1. Daniel, W.W. 1990. Applied Nonparametric Statistics. 2nd ed. PWS-KENT Publishing Company; Boston, MA.
2. Gibaldi, M. and P. Perrier. 1982. Pharmacokinetics. 2nd ed. Marcel Dekker, Inc.; New York.
3. Graham D.L. 1980. Acute avian herpesvirus infections. Current Veterinary Therapy VII, Small Animal Practice. R.W. Kirk, ed. W.B. Saunders Co.: 704–705.
4. Keating M.R. 1992. Antiviral agents. Mayo Clin Proc. 67(2):160–178.
5. Norton T.M., J. Gaskin, G.V. Kollias, B. Homer, C.H. Clark and R. Wilson. 1991. Efficacy of acyclovir against herpesvirus infection in Quaker parakeets. Am J Vet Res. 52(12):2007–2009.
6. Norton T.M., G.V. Kollias, C.H. Clark, J. Gaskin, R.C. Wilson and J. Coniglario. 1992. Acyclovir (Zovirax®) pharmacokinetics in Quaker parakeets, Myiopsitta monachus. J Vet Pharm Therap. 15:52–258.
7. Pharmacological description of Acyclovir. 1997. MOVA pharmaceutical corporation; Caguas, Puerto Rico.
8. Ritchie B.W. 1995. Avian Viruses. Chapter 7: Herpesviridae. Wingers Publishing, Inc.; Lake Worth, FL: 171–218.
9. Ritchie B.W., G.J. Harrison and L.R. Harrison. 1994. Avian Medicine: Principles and Applications. Wingers Publishing, Inc.; Lake Worth, FL: 874–884.
10. Rivere, J.E. 1999. Comparative Pharmacokinetics: Principles, Techniques and Applications. Iowa State University Press, Ames, Iowa.