Abstract
Stress has long been recognized as having a significant impact on animal health. An acute stress response is designed to help an organism deal with unexpected abnormalities that upset its homeostasis. Chronic stress, on the other hand, can impair immune function and reproductive cycles, among other things. There is also some question as to whether normal variants in an animal’s life cycle, like pregnancy, may also cause a stress response. Scientists have used fecal cortisol levels as a noninvasive measure of stress with success in many species. We used enzyme immunoassay (EIA) technology for fecal cortisol monitoring in six female okapi to determine whether pregnancy could be considered a physiologic stressor that affects the hypothalamic-pituitary-adrenal (HPA) axis and to begin establishing baseline fecal cortisol levels. We found in most cases (four of five pregnant females) no significant difference between pregnant and nonpregnant states. We did find significant differences in baseline cortisol levels between individuals. We also noticed a cortisol spike just prior to parturition that may have fetal origins.
Introduction
Okapi (Okapia johnstoni) are secretive, solitary individuals, endemic to the rainforests of Zaire (Democratic Republic of the Congo). It is impossible to create a captive habitat for okapi that mimics well the size or diversity of their home ranges. Okapi, therefore, may be particularly susceptible to the stresses of captivity and manipulation. They reproduce with difficulty in captivity at some institutions, particularly urban zoos, while they reproduce quite well at other institutions that have more restricted access to the public. A noninvasive method of monitoring stress in this species is particularly relevant and useful (L. Penfold, unpublished data).
The aim of this study was to establish baseline fecal cortisol concentrations in nonpregnant and pregnant female okapi and investigate whether pregnancy is considered a stressful event in the okapi.
Scientists and veterinarians have been using fecal corticosteroids as a noninvasive measure of stress in wild and captive exotic13 and domestic species for several years. Measuring stress in captive exotic species can be a valuable aspect to a preventive medicine regime since chronic stress has been shown to contribute to impaired immune system function and, hence, greater incidence or severity of disease.9 Monitoring stress may also help us to understand and remedy difficulties found in captive breeding of some species since stress may disrupt or impair reproductive cycles.8
Cortisol is secreted into the blood stream by the adrenal glands in response to ACTH.1 Cortisol is then, in part, filtered by the liver and secreted with the bile into the ingesta within the intestines. Older methods of evaluating HPA axis function by measuring cortisol levels in the serum or plasma are being supplanted or supplemented by methods of measuring fecal cortisol or its metabolites.11 Both enzyme immunoassay (EIA) and radioimmunoassay (RIA) technologies have been used successfully for fecal hormone monitoring in many domestic and nondomestic species.13 These measurements are potentially a much more accurate reflection of the true level of stress an individual experiences since drawing blood for serum or plasma measurements causes stress in and of itself. These assays (including this study) have been validated using ACTH stimulation trials, known stressor trials, and/or demonstrating parallelism between serial dilutions of fecal extracts and the standard curve.
Materials and Methods
Feces (∼10 g) were collected from six adult female okapi, three times weekly for 1.5–2 years and stored frozen (-20°C) until analysis. Fecal samples (0.5 g) were boiled in 10 ml of ethanol for 20 minutes to extract fecal metabolites4,13 and the supernatant recovered and dried completely. Dehydrated residue was re-dissolved in methanol, diluted (1:40) in PBS, and assayed in duplicate using a heterologous EIA with a polyclonal anti-cortisol antiserum and a cortisol conjugate (cortisol:horseradish peroxidase). Fecal extracts were diluted (1:40) for assay. Intra-assay coefficients of variation were <10%, and assay sensitivity was 3:9 pg/ml. Hormone concentrations are expressed as mass units of hormone per gram feces. Assays were validated by demonstrating parallelism between serial dilutions of fecal extract and the standard curve and by recovery of known hormone added to the standard (accuracy check). Previous studies validated the use of measuring cortisol in okapi feces by an ACTH challenge (Acthar gel, Rhone-Poulenc Roler Pharmaceuticals Inc, Collegeville, PA).12
Mean (SEM) fecal cortisol concentrations were determined for individual females, before and during pregnancy, except for one female (Animal 1) who was not bred during the course of this study. Differences in cortisol concentrations before and during pregnancy, and between females were examined using one-way ANOVA on ranks, to account for data that were not normally distributed, and Dunn’s method for all pairwise comparisons. Significance was determined at p<0.05.
Results and Discussion
We found no significant difference in stress levels as measured by fecal cortisol levels between pregnant and nonpregnant stages in 4/5 of the females. However, one female (Animal 3) had significantly higher cortisol concentrations during pregnancy than when nonpregnant (Table 1). Pregnancy-specific rises in plasma cortisol levels have been documented in late pregnancy in the bitch.9 In species of artiodactyls the case is not clear as to whether pregnancy is a physiologic stressor affecting the HPA axis. Some studies have shown that there is no significant difference between pregnant and nonpregnant states,5,14 whereas other studies have found significant differences in ACTH or cortisol levels between pregnant and nonpregnant states,2 with the higher levels recorded during pregnancy.
Table 1. Mean (±SEM) fecal corticosteroid concentrations (ng/g) in nonpregnant and pregnant okapi
|
|
Animal 1
|
Animal 2
|
Animal 3
|
Animal 4
|
Animal 5
|
Animal 6
|
|
n
|
157
|
119/110
|
57/41
|
28/133
|
43/130
|
33/56
|
|
Nonpregnant
|
23.5±1.5a
|
7.2±9.3b
|
8.6±0.5b
|
9.5±0.6b
|
18±0.7a
|
7.1±0.5b
|
|
Pregnant
|
|
9.3±0.4b
|
15±0.8b,c
|
11±0.3b,c
|
14±0.5a,c
|
7.9±0.4b
|
aRow and column means followed by different italicized superscripts abc differ p<0.05.
Regardless of whether or not pregnancy is considered a physiologic stressor in and of itself in okapi, it may still be useful to monitor cortisol levels during pregnancy. Stressful events that occur during pregnancy may detrimentally affect the fetus. Prenatal exposure to maternal glucocorticoids may negatively affect the fetal liver, causing reduction in birth weight and postnatal alterations in glucose hemostasis and function of the HPA axis.3,7 Fetal growth, cardiovascular function (particularly hypertension), endocrine status, ability to adapt to stress, and metabolism, in general, can suffer long-term effects from chronic, or elevated acute, exposure to maternal corticosteroids.
One female okapi (Animal 4), who has a history of early pregnancy losses, did not have significantly higher fecal corticosteroid concentrations before or during pregnancy compared to the other females (Table 1). Females with significantly higher fecal cortisol than the others were subjectively considered temperamentally more ‘stressed’ than the other females by the keeper staff.
A distinct (five-fold) rise in cortisol immediately prior to parturition was observed in one of our females (Animal 6, see Figure 1). It has been shown in other mammalian species that an increase in fetal cortisol is essential for the initiation of parturition.6 Thus, it is likely that we are detecting the excreted cortisol produced by the fetus, observable as a transient spike immediately before birth. If this is the case, then this is the first time that it has been shown that okapi fetuses provide the signal to stimulate the initiation of parturition. As the fetal release of cortisol is transient in nature, and feces were only collected three times weekly, it is not surprising that we missed detecting this prepartum rise in the other females.
Figure 1
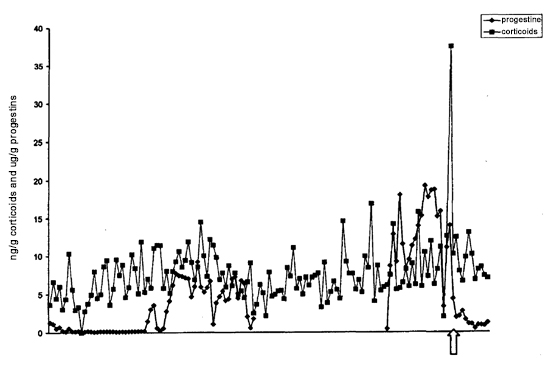
Fecal corticoids and progestin (ng/g) throughout pregnancy (Animal 6). Arrow denotes parturition
Wildlife species are subject to many sources of stress. The physiologic response to acute stress is a healthy, adaptive response and does not suppress the immune system.10 When stressors become chronic, however, the body’s normal stress adaptations have detrimental effects and can suppress the immune system, cause a decline in growth and atrophy of tissues, and interrupt the normal reproductive cycle.8,10 Sources of acute stress in the wild may include such things as predatory attacks or brief conflicts to establish social dominance. Sources of chronic stressors in the wild may include habitat encroachment, disease, pollution, climate extremes, and hierarchal social position. Captive animals have added sources of acute and chronic stress including immobilizations and restraint, transport, introduction of new individuals or separation from familiar individuals, unfamiliar environments or enclosures that fail to mimic crucial aspects of their natural habitat, confined spaces, new diseases, inadequate nutrition, boredom, and the constant presence of the viewing public and staff. Some of these may not have a direct effect on the HPA axis but may indirectly contribute to the animal’s overall stress levels.
Because chronic stress has a detrimental effect on immunity, monitoring fecal cortisol or cortisol metabolites is a potentially valuable component of preventive medicine protocols in a captive environment. Considering the wide range of possible stressors, and the great variability in baseline levels between individuals,5 high fecal cortisol levels may be relatively nonspecific, but can still be useful by alerting the veterinarian that something is causing undue stress to the animals. Many of our zoo animals only pass through our hands during their annual exams due to time constraints and the stress of immobilizations. Fecal monitoring causes no stress to our animals and can help to focus our attention on potential problems before more serious illness ensues.
Measuring fecal cortisol levels does have its disadvantages. Hormone metabolism and excretion differ between species so that different assays and protocols will need to be developed for each species of interest. Variability in data may also occur depending on time and circumstances surrounding collection, methods of storage, and processing protocols. Standards to govern these practices will need to be established and followed.
As workers in this particular aspect of the conservation biology field (zoo medicine), we work with nature’s creatures in an unnatural setting: captivity. Workers in our field have done much to try to make the captive environments of our charges more comfortable by making enclosures more like natural habitats, taking strides to reduce stereotypic behaviors, and improving nutrition and medical care. Quantifying stress via fecal cortisol measurement, identifying the stressors, and eliminating them can bring us even closer to providing a comfortable, healthy environment for our captive animals. This concern becomes more pertinent with animals, like the okapi, that are adapted to secluded and/or large home ranges.
Literature Cited
1. Axelrod J, Reisine T. Stress hormones: their interaction and regulation. Science. 1984;224:452–459.
2. Bell ME, Wood CE, Keller-Wood M. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domest Anim Endocrinol. 1991;8:245–254.
3. Brown JL, Wasser SK, Wildt DE, Graham LH. Measurement of fecal estrogen and progesterone metabolites for assessing ovarian activity in felids. Biol Reprod. 1994;51:776–786.
4. Bubenik GA, Schams D, White RG, Rowell J, Blake J, Bartos L. Seasonal levels of metabolic hormones and substrates in male and female reindeer (Rangifer tarandus). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;120:307–315.
5. Challis JFG, Olson DM. Parturition. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. Vol. 2. New York, NY: Raven Press; 1988:2177–2216.
6. Concannon PW, Butler WR, Hansel W. Parturition and lactation in the bitch: serum progesterone, cortisol, and prolactin. Biol Reprod. 1978;19:1113–1118.
7. Karsch FJ, Battaglia DF, Breen KM, Debus N, Harris TG. Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress. 2002;5:101–112.
8. Maddock C, Pariante CM. How does stress affect you? An overview of stress, immunity, depression, and disease. Epidemiol Psychiatr Soc. 2001;10:153–162.
9. Moberg GP, Mench JA. The Biology of Animal Stress. New York, NY: CABI Publishing; 2000.
10. Mostl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol. 2002;23:67–74.
11. Othen L, Jarsky T, French J, Crichton E, Loskutoff N, Bennett C. Correlating ethological and physiological parameters as indicators of well-being in okapi (Okapia johnstoni): ACTH challenge, patterns of excretion, and impact of introductions. In: Proceedings from the Second International Symposium of Assisted Reproductive Technology. (ART) Cons Genetic Man Wildl. 2002; Henry Doorly Zoo, Omaha, NE. 73–79.
12. Stead SK, Meltzer DG, Palme R. The measurement of glucocorticoid concentrations in the serum and faeces of captive African elephants (Loxodonta africana) after ACTH stimulation. J S Afr Vet Assoc. 2000;71:192–196.
13. Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, et al. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275.
14. Wintour EM, Blair-West JR, Brown EH, Coghlan JP, Denton DA, Nelson J, et al. The effect of pregnancy on mineralo- and glucocorticoid secretion in sheep. Clin Exp Pharmocol Physiol. 1976;3:331–342.