Development of a Tool for Assessing and Managing the Risk of Avian Mycobacteriosis During Avian Translocation
Abstract
Infectious diseases potentially impact the health of both individuals and populations. For diseases that are chronic, contagious, and elusive, as is the case with avian mycobacteriosis, risks may be magnified. This disease is problematic worldwide in both captive and wild populations. Once infected, an individual is capable of protracted shedding of viable organisms into the environment. Diagnosis is hampered by the lack of reliable antemortem tests, slow-growing cultures, and an inability to effectively treat the disease. Therefore, the best approach is to minimize introduction of the organism into an avian population, ensuring a source free of disease for both captive breeding and reintroduction programs. Due to ambiguity between institutions’ assessment of risk and the nature of this disease, many facilities are reluctant to accept birds from sources reporting a collection history with Mycobacterium avium. Review of the literature, case reports, and diagnostic reviews demonstrate the pervasive nature of the disease, but in no documentation are the complete risk factors of exhibit, species, and population systematically analyzed by any standardized method. This paper outlines an easily understood risk assessment tool, created by a multidisciplinary group of experts, that allows managers and health experts to cooperatively evaluate risk factors associated with the spread of avian mycobacteriosis within and between institutions and into wild populations.
Introduction
Avian mycobacteriosis, or tuberculosis, is a pervasive disease issue in many avian species. Although the bacterial genus is ubiquitous as several species are found in soil, the disease in birds is most commonly caused by M. avium or M. genavense. Birds are infected by fecal–oral transmission within a contaminated environment. Feral bird species are considered contributors to outside exhibit contamination or exhibit substrates. Virtually all avian orders have had representative infections, but it is more often diagnosed in Anseriformes, Gruiformes, Galliformes, and Psittaformes.1-3 In some particular species, mycobacteriosis has been found as a prevalent cause of death, such as in Micronesian kingfishers (Halcyon cinnamomina cinnamomina) (11% of deaths as reported in 1996) and white-winged wood ducks (Cairina scutulata) (80% of deaths as reported in 2003).4,5
While avian mycobacteriosis may cause mortality for some individual animals, exposure to this organism or an exhibit with historic contamination does not necessarily result in death or disease. Unfortunately, many animal movement decisions are made based on the perception of risk since there is not much data available. As a result, many individuals that have extremely low risk of spreading disease may be unnecessarily euthanatized or quarantined. Risk assessment methodology helps integrate science into decision-making policy through a standardized approach to assessing the risk of disease. A multi-stakeholder workshop was held at Lincoln Park Zoo in order to provide a forum for open discussion between veterinarians and managers regarding animal movements and the risk of spreading avian mycobacteriosis. As a result of the workshop, a semi-quantitative risk assessment tool, useful for both veterinarians and managers, was developed. This effort has the potential to positively affect many individual species as it is a demonstrated problem industry wide. The disease significantly impacts captive breeding of many endangered species; common examples include increased mortality of breeding stock (e.g., Micronesian kingfishers), and decreased compliance with programmatic breeding recommendations (e.g., sunbittern [Eurypyga helias] Guam rails [Rallus owstoni]). When faced with this disease, managers and veterinarians are forced to make decisions for their collection as well as AZA®-sponsored programs (SSP®, PMP, TAG, Avian SAG, and Reintroduction SAG) using information that is vague, contradictory, and not standardized within the industry. This tool, endorsed by both veterinarians and managers, offers one standardized approach to dealing with this issue.
Methods
Risk assessment follows a formal process, the phases of which include: 1) outlining the generic pathway of concern (in this case avian animal movement from point A to point B); 2) identification and weighting of risk factors specific to the pathway; 3) establishing evaluation or grading criteria by which risk factors (qualitative vs. semi-quantitative vs. quantitative) are ranked; 4) describing the relationship between factors in order to combine them all to calculate total measurement of risk; and 5) validation of the process. In this case, the source institution including enclosure, management and veterinary service contributions; quarantine and testing; shipment method; and post-shipment environment are all considered in the model’s pathway.
Risk factors for each of the areas above were specifically defined (i.e., prevalence in the source population, likelihood of being infected by wildlife at all stages, sensitivity of the diagnostic test, etc.). Each factor was then weighted according to its relative importance to overall risk of disease introduction or transmission; this step was a major part of the expert discussion during the first workshop. The total risk for each stage is calculated and the stages combined for overall risk characterization from source through destination—the lower the overall score, the lower the risk. A special data collection form was created using Microsoft Access© to take the users through this process in a step-by-step, transparent, organized manner.
The method described above is known as a semiquantitative assessment. Internationally accepted methodology exists for conducting semiquantitative risk assessments for issues surrounding animal health and animal movements.6-8 Two different, but equally appropriate examples, are available online: the USDA protocol for assessing Johne’s disease risk in cattle (http://www.state.vt.us/agric/VTCHIP/vchipchecklist.pdf) (VIN editor: This link was not accessible as of 1-28-21), and the European Union financial investment risk assessment method (http://www.ltbcweb.com/cipratech/squat.html) (VIN editor: This link was not accessible as of 1-28-21). Semiquantitative calculations, using ranked categoric data (equivalent to those used to determine grade point average), are commonly used in cases where there is minimal quantitative data available—clearly, this is the case with avian mycobacteriosis.
Based upon final score, an individual animal will be placed into a risk category “red,” “yellow,” or “green” (Figure 1). In general, the red category means movement of the animal is at high risk for transferring a mycobacteriosis-positive individual, while those placed in the yellow category are acceptable with some reservations or issues needing to be addressed. Those in the green risk category have minimal risk of transferring the disease to the receiving institution or population. Standardized management recommendations and guidelines are being developed for each risk category (red, yellow and green) to assist managers with making decisions regarding animal movements once their risk has been assessed.
Figure 1. Schematic flow diagram of avian mycobacteriosis risk assessment process
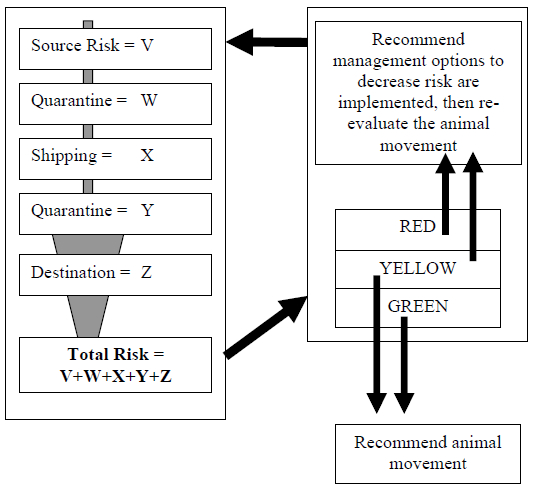
Figure 2. Diagnostic test decision tree for avian mycobacteriosis
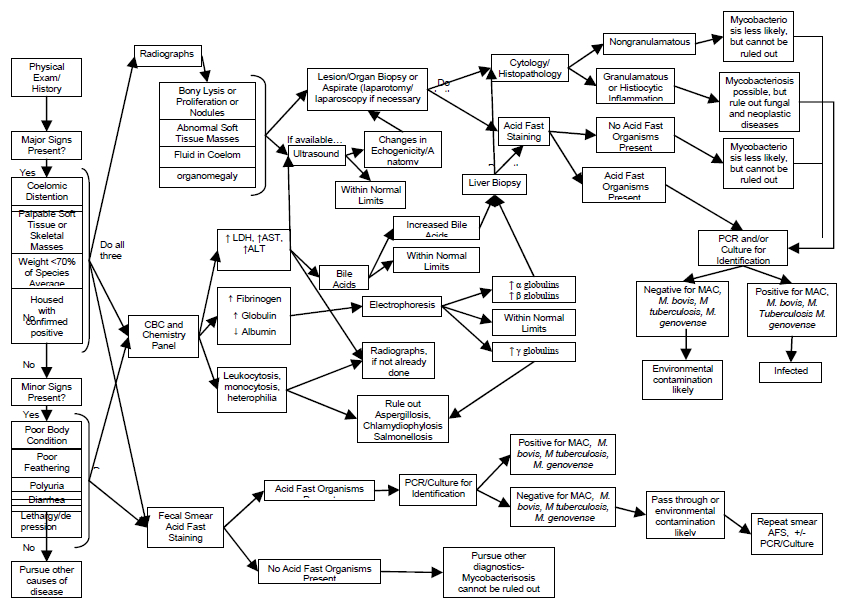
Reading from left to right, minimal recommended screening procedures include physical examination, radiographs, CBC, blood chemistry, and acid-fast stained fecal smear. Further diagnostic testing recommendations, with interpretations, may be considered by following the flow diagram.
Results and Discussion
The expert elicitation workshop resulted in the inclusion of the following risk factors into the model (weights of each factor not included):
- Immune status of the bird to be shipped (individual medical history)
- Age class
- Parent history with respect to TB (risk of vertical transmission)
- Exposure history to positive birds at enclosure, building or run levels (horizontal transmission)
- Exposure history to wild birds
- Enclosure maintenance and hygiene
- Husbandry and enclosure environmental factors such as ventilation, water, substrate, equipment, food, and lighting
- Exposure to people (personnel, contract workers and public) and biosecurity practices
- Quarantine management, personnel, biosecurity, including cleaning and disinfection practices, and degree of isolation and animal/people flow patterns
- Diagnostic testing—minimal screening recommendations and weighted values of other testing methods
- Shipping factors including both air and ground transport, environmental stress, and personnel/equipment hygiene and disinfection
- Post-shipment quarantine/isolation and testing protocols
- Destination factors such as mycobacteriosis history and status in recipient population
- Impact of AZA program recommendations in captive settings
- Impact on potential reintroductions (e.g., IUCN guidelines, post-release monitoring, etc.)
Acknowledgments
Funds for this work were provided by the Conservation Endowment Fund of the American Zoo and Aquarium Association. Working Group Members: Kim Smith, Milwaukee County Zoo; Joanne Earnhardt PhD, Lincoln Park Zoo; Michael Mace, San Diego Wild Animal Park; Robert Seibels, Riverbanks Zoo; Joe Barkowski, Sedgwick County Zoo; Patricia Shreve, Tracy Aviary, Mark Myers, Audubon Nature Institute/Audubon Zoo; Zoli Gymesi, Louisville Zoo; Don Neiffer, Disney’s Animal Kingdom; Lisa Tell, UC Davis; Randy Junge, St. Louis Zoo; Becky Manning, University of Wisconsin; Gary Riggs, Akron Zoo.
Literature Cited
1. Dorrestein, G.M. 1997. Infectious diseases—bacteriology. In: Altman, R.B., S.L. Clubb, G.M. Dorrestein, and K. Quesenberry, eds. Avian Medicine and Surgery. W.B. Saunders, Philadelphia, PA. Pp. 275–276.
2. Klein, P.N. 2002. Avian tuberculosis. Am. Assoc. Zoo Vet. Infect. Dis. Comm.: 1–12.
3. Phalen, D.N. 2000. Avian mycobacteriosis. In: Bonagura, J.D., ed. Kirk’s Current Therapy XIII Small Animal Practice. W.B. Saunders, Co., Philadelphia, PA. Pp. 1116–1118.
4. Reed, R., R.J. Montali, D.K. Nichols, and S.B. Citino. 1992. Clinical challenge: hepatomegaly caused by nontuberculous mycobacteriosis. J. Zoo Wildl. Med. 23: 383–386.
5. Silva-Krott, I., M.K. Brock, and R.E. Junge. 1998. Determination of the presence of Mycobacterium avium on Guam as a precursor to reintroduction of indigenous bird species. Pac. Conserv. Biol. 4: 227–231.
6. Van der Logt, P., D. Vose, and S. Hathaway. 1997. Risk assessment model for human infection with the cestode Taenia saginata. J. Food Protection. 60: 1110–1119.
7. Vose, D. 1997. Risk analysis in relation to the importation and exportation of animal products. Sci. Tech. Rev. 16.
8. Vose, D. 2000. Risk Analysis: A Quantitative Guide. J Wiley, Chichester, England.