Adrenal Activity Assessed by Fecal Corticoids and Male Reproductive Traits in Three South American Felid Species
Abstract
Reproductive success in captive small-sized cats is very low or inconsistent in Latin American zoos, and few of the captive males (∼16%) have ever sired offspring.7 Although gonadal function is controlled by a complex interaction of biological factors, poor husbandry conditions in zoos may be partly responsible for impaired reproductive function in captive populations. In particular, inadequate nutrient intakes and an inadequate environment (physical and/or social conditions) can compromise reproduction through behavioral and/or physiological mechanisms.1,4,7 Most zoos manage small-sized cats identically, without considering possible physiological and psychological differences among species. It is quite possible that optimal conditions for one species are not appropriate for another. Thus, in this study, we compared reproductive activity and fecal corticoid levels (as an indicator of stress) among male ocelots (Leopardus pardalis), tigrinas (Leopardus tigrinus), and margays (Leopardus wiedii). Specifically, we 1) evaluated testicular volume and seminal characteristics; 2) assessed the stress response to captive conditions and experimental manipulations; and 3) calculated correlations between fecal corticoid levels and sperm production and quality.
Three males of each species were housed individually and fed a meat-based diet with vitamin and mineral supplementation. Each male was submitted to monthly reproductive evaluations over a 14-month period. After 12–24-hour fasting, anesthesia was induced in ocelots with tiletamine-zolazepam (10 mg/kg, IM, Telazol, Fort Dodge Laboratories, Inc., Fort Dodge, IA) and in tigrinas and margays with a combination of ketamine HCl (20 mg/kg, IM, Ketaset, Fort Dodge Laboratories, Inc., Fort Dodge, IA) and xylazine (1 mg/kg, IM, Rompun, Mobay Corp., Shawnee, KS). Semen collection and analysis followed standardized protocols.3 The reproductive parameters analyzed were testicular volume, ejaculate volume, total number of sperm cells/ejaculate, sperm motility and status, and sperm morphology. Fecal samples were also collected once/week during the experimental period for analysis of corticoid metabolite concentrations. Steroids were extracted from feces as described previously,2 and corticoids quantified using a previously validated double-antibody 125I-corticosterone assay (ICN Biomedicals Inc., Costa Mesa, CA).6 Assay sensitivity, based on 80% of maximum binding, was 12.5 ng/ml. Intra- and inter-assay coefficients of variation were <10%. Hormonal data are expressed “per g of wet fecal weight.” Peak and baseline concentrations were calculated as previously described.5 Differences among species in endocrine and reproductive data were determined by a one-way analysis of variance (ANOVA) followed by Duncan’s new multiple range tests.
Mean values (± SEM) for body weight, testicular volume, ejaculate characteristics, and fecal corticoid levels for each species are presented in Table 1. Spermatogenic activity, as determined by both quantitative and qualitative assessments, was higher in the ocelot, whereas margay males exhibited the lowest sperm production and highest rates of primary spermatogenic defects, mainly abnormal acrosomes and sperm head malformations. Tigrinas had intermediate values for almost all of the characteristics analyzed, with the exception of total number of sperm cells. Surprisingly, tigrina males produced numbers of sperm cells/ejaculate similar to that for ocelot males. When analyzed on a “per unit of testicular volume” basis, tigrinas produced approximately five- and sixfold more sperm cells than margays and ocelots, respectively. In comparison to previous assessments for these same three species,3,7 an improvement in reproductive characteristics was noted, particularly those related to quantitative semen measurements. These findings may be the result of feeding an improved diet enriched with vitamins and minerals, which began 5 months prior to initiation of this study. Frequency of sperm collection, however, may also play a role, since significant (p<0.05) correlations were observed between number of previous sperm collections and 1) testicular volume (r=0.71, r=0.47, r=0.52); 2) ejaculate volume (r=0.45, r=0.59, r=0.51); and 3) total number of sperm cells/ejaculate (r=0.36, r=0.34, r=0.47) in tigrinas, margays, and ocelots, respectively.
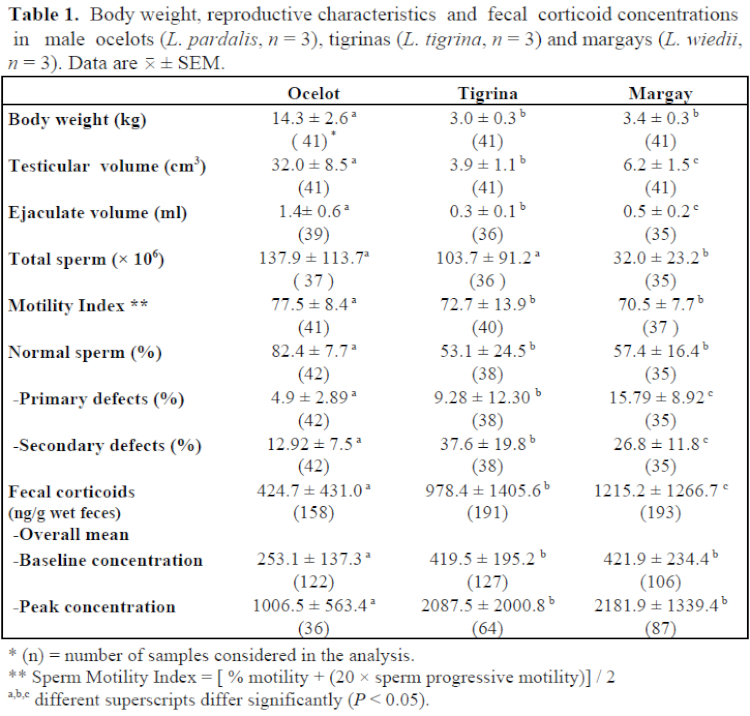
Fecal corticoid levels differed significantly among species. Overall mean, baseline, and peak corticoid levels were lower (p<0.05) in ocelots than in the other two species. All three species responded to different “stressors” (pre-anesthetic fasting, anesthesia, electroejaculation, and “usual” captive stressors such as routine caretaking procedures and climatic changes) by excreting higher levels of corticoids. However, tigrina and margay males seemed to be more sensitive than ocelot males. A significant (p<0.05) seasonal effect was observed only in margays, with higher levels observed during winter (June–August) in comparison to summer (December–February). A negative correlation between corticoid levels and ambient temperature (r=0.52; p<0.05) was also found in margays. Corticoid excretion in ocelots and tigrinas tended to increase with ambient temperature increments, although a significant correlation was observed only in the ocelot (r=0.46; p<0.05). For all data combined, correlation coefficients between corticoid levels and 1) body weight (r= -0.43), 2) testicular volume (r= -0.40), 3) total sperm cells/ejaculate (r= -0.20), and 4) % of normal sperm cells (r= -0.32) were all significant (p<0.05).
In conclusion, although maintained under similar environmental and husbandry conditions in captivity, different small-sized cat species appear to respond differently to various acute stress factors. There also were species differences in basal adrenal activity as determined by fecal corticoid analysis. Thus, fecal corticoid measurements may provide us a means of monitoring stress responses associated with the captive environment. Finally, although this study was not specifically designed to establish cause–effect relationships, there was a modest relationship between higher fecal corticoid concentration and impaired spermatogenic function in small felids kept in captivity.
Acknowledgments
The authors thank the Curitiba Zoo and Itaipu Binacional for providing animals and assistance with sampling. We are also grateful to Laura H. Graham for the radioimmunoassay technical assistance. This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), the New Opportunities in Animal Health Sciences (NOAHS) Center and Friends of National Zoo (FONZ), British Airways, and The Philip Reed Foundation.
Literature Cited
1. Carlstead, K., J.L. Brown, and W. Strawn. 1993. Behavioral and physiological correlates of stress in laboratory cats. Appl. Anim. Behav. Sci., 38: 143–158.
2. Graham, L.H. and J.L. Brown. 1996. Cortisol metabolism in the domestic cat and implications for non-invasive monitoring of adrenocortical function in endangered felids. Zoo Biology. 15:71–82.
3. Howard, J.G. 1993. Semen collection and analysis in carnivores. In: Fowler M.E. (ed.). Zoo & Wild Animal Medicine: Current Therapy 3. W.B. Saunders Co., Philadelphia. Pp. 390–399.
4. Mellen, J. 1991. Factors influencing reproductive success in small captive exotic felids (Felis spp.): a multiple regression analysis. Zoo Biology. 10:95–110.
5. Morais, R.N., N. Moreira, W. Moraes, R.G. Mucciolo, O. Lacerda, M.L.F. Gomes, W.F. Swanson, L.H. Graham, J.L. Brown. 1996. Testicular and ovarian function in South American felids assessed by fecal steroids. Proc. American Association of Zoo Veterinarians. Pp. 561–565.
6. Stillwell, H.J., J.L. Brown, and L.H. Graham. 1996. Assessment of a commercially available radioimmunoassay for the detection of fecal cortisol metabolites in several non-domestic felid species. Proc. American Association of Zoo Veterinarians. Pp. 582–583.
7. Swanson, W.F., D.E. Wildt, R.C. Cambre, S.B. Citino, K.B. Quigley, M.D. Brousset, R.N. Morais, N. Moreira, S.J. O’Brien, and W.E. Johnson. 1995. Reproductive survey of endemic felid species in Latin American zoos: male reproductive status and implications for conservation. Proc. Joint. Conf.: AAZV/WDA/AAWV. Pp. 374–380.