Factors Influencing Interpretation of Indirect Testing Methods for Tuberculosis in Elephants
Abstract
Serologic and other laboratory tests (such as BTB, ELISA and gamma interferon) are often used in conjunction with the intradermal tuberculin test to detect tuberculosis (TB) in animals.2 The skin test is considered the “gold standard” in domestic cattle and humans, and the BTB test has been highly rated for use in cervid species. However, these indirect methods for TB diagnosis have not been proven valid in most exotic species susceptible to the Mycobacterium tuberculosis complex (which includes M. bovis) infection. In addition, many of the tuberculin skin testing methods used in exotic species are not uniform in terms of tuberculin type(s) and sites used and interpretation of the end points.3
Recently, cases of TB due to M. tuberculosis have occurred in several elephant collections located in the Midwestern and western United States. In these cases, intradermal and serologic tests did not correlate well in elephants that were culture-positive for M. tuberculosis.1 This led to the development of guidelines to control TB in elephants in which culturing the trunk for TB organisms is the key diagnostic procedure (U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Animal Care, Guidelines for the Control of Tuberculosis in Elephants, November 1997). However, intradermal and serologic testing have been encouraged in order to collect further data as a research effort to establish the validity of these ancillary tests in elephants.
In April of 1997 a female Asian elephant (Elephas maximus, elephant #1) at the National Zoological Park had a 3-cm reaction to a ppd-bovis intradermal skin test administered while being clinically evaluated for an intermittent low-grade cough. A BTB test, taken the day of the tuberculin test (4/27/97), was negative. A comparative test 9 days after the initial intradermal test (5/2/97) with balanced ppd-bovis and ppd-avium tuberculins showed a 3:1 avium:bovis reaction indicating the original skin test reaction was most likely from cross-reactivity to atypical mycobacteria. A blood sample taken on the same day as the comparative test (5/2/97), however, came back with a strongly positive ELISA test for M. tuberculosis (0.2–0.5 = weakly positive to strongly positive) in which only M. tuberculosis antigens were used (Fig. 1). Using banked serum samples acquired in 1995, 1996, and 1997, further ELISA testing was performed using antigen from M. tuberculosis and M. avium (Fig. 2). All samples acquired prior to and including the day of the original skin test (4/23/97) were in the ± zone but the sample taken 9 days after tuberculin testing was strongly positive. The responses to M. avium antigen were greater than the responses to M. tuberculosis antigen.
| Figure 1 | 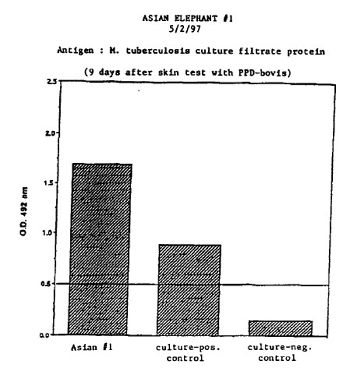
ELISA |
|
| |
| Figure 2 | 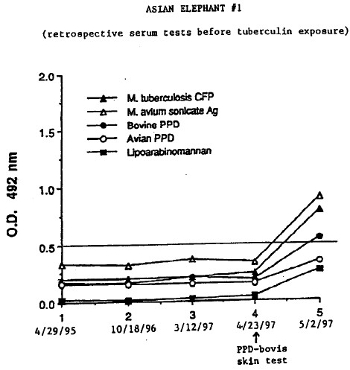
ELISA |
|
| |
Subsequent blood samples taken from elephant #1 taken at monthly intervals in 1997, showed a continued rise of the ELISA test well into the positive zone over the next month followed by a steady decline towards the negative zone over the following 2 months (Figs. 3a and 3b). ELISA tests were performed on two other female Asian elephants (elephants #2 and #3) and one female African elephant (Loxodonta africana, elephant #4), using stored serum (no tuberculin testing during this period), serum taken the day the animals were given comparative tuberculin skin tests, and, subsequent to the comparative tuberculin testing (elephants # 2 and #3). As with elephant #1, the ELISA readings rose in elephants #2 and #3 after tuberculin testing with elephant #2 approaching the positive zone (data not shown). Elephant #4 was not skin tested and ELISA readings remained in the normal zone (data not shown). In addition, elephants #1 and #4 tested “positive” for M. bovis antigen using the BTB test.
| Figure 3A | 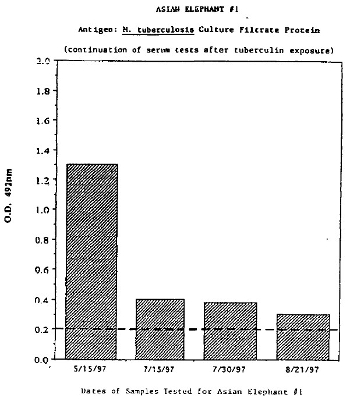
ELISA |
|
| |
| Figure 3B | 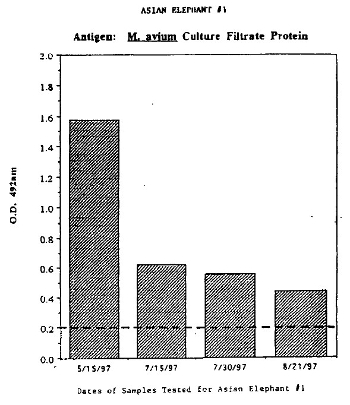
ELISA |
|
| |
Elephant #1 recovered from its vague illness after antibiotic treatment. Trunk cultures performed on the animal three times within a 7-day period were all negative for Mycobacteria sp. as were trunk cultures performed on the other three elephants during the course of the indirect testing. All six of the elephant keeper staff had negative tuberculin tests within the same time period.
This experience demonstrated some of the pitfalls in trying to rely on ancillary tests for determining the tuberculosis status of an elephant collection. None of the animals in our herd have had any previous exposures to other elephants for the past 10 years. The initial reaction to the ppd-bovis in elephant #1 was attributed to cross-reactivity to non-tuberculous Mycobacteria as determined by the comparative skin test. The “positive” ELISA and BTB tests performed on serum taken after the skin tests in elephant #1 were attributed to tuberculin exposure. Taken out of context, this finding may have placed the animal in jeopardy if interpreted as positive for tuberculosis.
It is beneficial to perform comparative testing in elephants and even to initiate testing using the comparative procedure. It is also important when using ELISA and other serologic tests to request that both M. avium and M. tuberculosis (and/or M. bovis) antigens be compared in the testing procedure. Elephants are constantly exposed to saprophytic Mycobacteria sp. and can be readily colonized by these “atypical” organisms by their habit of trunk “bathing” with water and soil. Most of the ELISA reactivity in the tests shown above (Fig. 3b and not shown), were more intense with the M. avium antigens, supporting the cross-reactivity interpretation of the comparative test in elephant #1. In addition, from our temporal studies of stored sera and from subsequent blood sampling before, at the point of, and after skin testing, it was evident that tuberculin exposure influences the ELISA and to some extent the BTB tests and can cause false-positive results in elephants.
As of April 1998 (approximately 1 year after the initial testing), all elephants are well and the cough in elephant #1 has not recurred. The temporal serologic studies were continued to determine if, and at what point, the ELISA readings return to pre-tuberculin exposure levels.
In this time of heightened activity of testing for TB in elephants, it will be important to continue studies of the indirect tests and correlate the findings with the culture results. This should result in establishing more reliable tests for diagnosing TB in the earlier (non-shedding) stages of the disease and for prognostic purposes.
Acknowledgments
This work was supported in part by the Friends of the National Zoo (FONZ) Kumari Elephant Fund. We thank Dr. Sang Nae-Cho, Colorado State University, National Veterinary Services Laboratories’ mycobacteriology staff, Ames, IA and Dr. Charles Thoen, University of Iowa for laboratory assistance, and the National Zoo’s elephant staff for special assistance while evaluating and testing the elephants.
Literature Cited
1. Binkley M. Tuberculosis in captive elephants. In: Proceedings of the American Association of Zoo Veterinarians. 1997:116–119.
2. Mikota S, Maslow J. Theoretical and technical aspects of diagnostic techniques for mammalian tuberculosis. In: Proceedings of the American Association of Zoo Veterinarians. 1997:162–165.
3. Montali RJ, Hirschel PG. Survey of tuberculin testing practices at zoos. In: Proceedings of the American Association of Zoo Veterinarians. 1990:105–109.