Guidelines for Veterinary Assistance During the Reproductive Process in Female Elephants
Abstract
In February 2000, a group of European zoo veterinarians met at Tierpark Hagenbeck, Hamburg to evaluate a questionnaire about 31 parturitions in Asian elephants. The results were presented at the 40th International Symposium on Diseases of Zoo and Wild Animals.1 These results were combined with the experiences of some North-American zoo veterinarians, which resulted in the protocol presented in this paper. This protocol may serve as a guideline for institutions that wish to breed elephants. The proper application of the recommendations given in these guidelines should increase the reproductive success in elephants. It is the moral obligation of everyone who is responsible for the management and breeding of elephants to consider utilizing the guidelines as they apply to their situation and to collect data that may help increase our knowledge.
The breeding process in elephants requires monitoring of several parameters in both males and females. The most crucial parameters are the determination of the estrous cycle through progesterone and, perhaps, LH assay, evaluation of the genital tract in both sexes,2,3 determination of the number of fetuses, and finally, parturition. The first part of this paper will mention briefly the tools that can be used in female elephants to achieve these goals. The second part describes a protocol for veterinary intervention in elephant parturition.
Monitoring and Assisting Female Reproduction in Elephants
Determination of Estrous Cycle
Monitor blood (progesterone)4 or urine (pregnanetriol)5 at least biweekly during the luteal phase; if more accuracy for estrous prediction (e.g., for breeding) is required, monitor every week. Monitor every week during the follicular phase. When using the urine test, be aware that the pregnanetriol concentration in the urine is compared with creatinine. If the creatinine level is too low, a new sample should be submitted. Even when monitoring the cycle by urine-pregnanetriol, much effort should be made to train all elephants to allow blood sampling.
Breeding
The occurrence of mating is not an indicator of impending ovulation; some animals mate even during advanced pregnancy or outside the estrous period. Though not yet documented, we suppose that fertilization can only result from mating during an estrous preceded by the pre-ovulatory (= second) LH-peak. The fertile period is restricted to the time around the rise in progesterone. For the prediction of the next ovulation, follicular phase length has to be determined and, therefore, it is important to know the moment of the preceding fall of progesterone/pregnanetriol. For better accuracy, weekly samples throughout the cycles have optimal predictive value. LH monitoring daily during the non-luteal phase is necessary for artificial insemination programs. Determination of the post-luteal-phase (= first) LH-peak can help predict the first opportunity for (natural) breeding, since the interval between both LH-peaks is rather constant.6
Pregnancy Confirmation
Immediate increase of progesterone/pregnanetriol around the time of mating is suggestive of the correct timing for breeding. Preliminary experiences with the use of an LH-test in Asian elephants have shown that many Asian elephants have a 1-day drop in progesterone and then a rapid rise the day following the LH surge.7 Continuation of high progesterone/pregnanetriol level continuing for at least 16 weeks after mating is highly suggestive of pregnancy. Ultrasonographic examination at 8–20 weeks after mating allows easy visualization. Between 10 and 20 weeks, the larger mature animals may need to lay down on its side for reliable ultrasonographic examination of the uterus. Uterine vascularization can be visualized to determine viability of the fetus, to exclude embryo absorption and mummification. At 6 months post breeding pregnancy can be confirmed by elevated serum prolactin concentration.7
Monitoring at the End of Gestation
Progesterone assays are recommended every other day from week 89 to 91. From week 91 (637 days) daily progesterone assays are recommended and as soon as progesterone starts to decrease, sample twice daily. Daily monitoring of progesterone is only possible if you have a nearby facility that runs these assays on a daily basis. Many labs do not have sensitivity for levels of progesterone (P4) in elephants 1–3 days prior to delivery. Loss of mucous plug (not seen in many facilities) is another indication of impending delivery.
Behavioral changes may be observed in the elephant that is due for calving (short periods of separation from the group, beating the vulva with the tail, frequent production of small-sized feces and small quantities of urine.8 Group members may react differently (vocalizations, restlessness) 24-hour observation including the use of a (time-lapse) video recorder starting in week 85 may add to information. The most relevant information for later decisions is the moment the amniotic sac ruptures. It is interesting to investigate the implications if only the allantois membrane ruptures. In most cases (75%) birth is seen within 2 hours of rupture of fetal membranes.1 Development of mammary gland and production of milk shortly before birth is often seen. Pre-and post-parturition ventral edema may be noticed.
Preparations for Calving
If possible, store some colostrum (freezer) or store plasma obtained from the dam in weeks prior to parturition. Have artificial milk available (hand rearing has been done at Emmen Zoo and Berlin Zoo). Check restraint chains and fixation points. Check the stable and place bars where a calf could possibly escape. Take out all obstacles. Make sure there is a good stock of bran or saw dust to be used on a concrete floor as soon as the calf is born. This will absorb much of the amniotic fluids and prevent the animals from slipping on the wet floor.
Supplies to have on hand include two or three pairs of keeper-gloves to get a better grip on the wet, slippery calf when needed, plastic hose pipe (with pump, if necessary) for rectal cleaning with lukewarm water, three birth-chains with proper handles (two for the legs, one for trunk or tail), find a way to avoid back sliding when manual extraction (vaginal vestibulotomy is required). Drugs to be kept in store include Betadine iodine, oxytocin, Ca-borogluconate, estrogen, lidocaine, xylazine, azaperone, atipamezole, doxapram, and oxygen. With the limited knowledge that is available now, we suppose that normal calving should take place within 2 hours after rupture of membranes (release of fetal fluids).
The moment when veterinary intervention should start is still to be determined. At this time, we have to rely on the data known from 20 cases.1 Fifteen cows delivered within 2 hours: 12 live and three dead calves. One cow delivered a healthy calf after 15 hours. Four cows delivered after >25 hours: one hypocalcemia (alive), three dead calves (2x episiotomy). Normal calving should take place within 48 hours after decrease of progesterone to below 0.1 ng/ml.
Not much validation has been done regarding the interval between the moment of complete absence of detectable blood progesterone level and the birth of a live calf. Efforts should be made to collect more data about this very important moment. In a few cows, progesterone levels in the last 8 weeks of pregnancy may be near baseline and determination of this event is difficult. However, most cows have a 50% drop in a 12–24-hour period and then drop to baseline levels in the next 12–24-hour period.9 Figure 1 shows this correlation between progesterone drop and time of birth similar as to what was demonstrated by Brown.9 There are other exceptions to this where a couple of cows have remained above baseline levels and parturition occurred.
| Figure 1 | 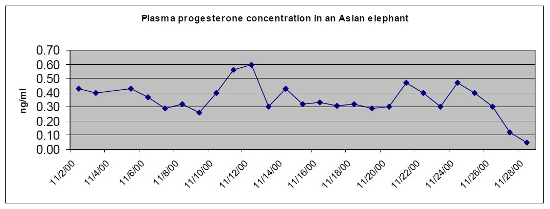
Plasma progesterone concentration in an Asian elephant. The calf was born at the end of the day when the progesterone level had dropped below the detection level of 0.1 ng/ml. (Rotterdam Zoo 2001.) |
|
| |
Guidelines for Veterinary Assistance at Parturition
The normal birth process in elephants has been poorly documented. Data of most cases has been kept as medical records at different institutions. The recommendations made here are based on the personal experience of the authors, results of the European survey,1 and data from the literature8.
To determine the moment when calving starts, two parameters are essential, the blood progesterone level and the relaxation of the cervix, monitored by ultrasonographic examination. One of the authors has used a commercial kit (Mare Foaling Predictorkit, Animal Health Care Products, Vernon, CA, USA), that measures the calcium level in milk to predict the time of parturition. This requires daily milk samples at the end of gestation.
Progesterone
The sensitivity of the equipment and the time needed to run the assay are the bottleneck for using the progesterone concentration as a reliable tool. Today many human hospitals use advanced equipment with a minimal detection level of 0.1 ng/ml that can be determined in less than 2 hours.
Ultrasonography
To use this technique as a reliable tool, it is indispensable for the veterinarian to gain experience long before the elephant birth occurs. This will enable the veterinarian to distinguish the relaxation of the cervix from the normal cervix. A 3.5 or 5 MHz probe can be used transrectally to visualize these changes. During the last 2 weeks of gestation, the mucous that is present in the vagina during gestation will be discharged gradually. This is a clear indication of impending parturition.
Once the birth process starts, two situations may be encountered: delivery has started with or without spontaneous rupturing of the fetal membranes. In the European study,1 13 out of 20 (65%) elephants delivered the calf within 5 minutes after rupture of the amniotic sac and another two animals within 2 hours. From the remaining five animals, one elephant suffered from hypocalcemia (Rotterdam Zoo, unpublished data) and three animals produced a dead calf (including two vaginal vestibulotomy cases). The different findings (closed cervix or open cervix) of transrectal ultrasound investigations illustrate that veterinary intervention may be required if parturition does not take place within 2 hours after rupture of the fetal membranes.
No Rupture of Fetal Membranes Noticed
Generally, the blood progesterone level drops from the maintenance level to baseline level (<0.1 ng/ml) in the last 2–3 days of gestation. It is expected that generally the calf will be born within 48–72 hours after the blood progesterone concentration has dropped to baseline level. However, one of the authors found that most calves are born alive and active within 3–5 days after progesterone drops to baseline in over 15 parturitions he attended and another 10 on which he consulted. Some births took place 7–8 days following drop (as long as the cervix is not dilated and labor had not been initiated).
If the calf is not born naturally 24 hours after blood progesterone has dropped to baseline level, rectal palpation and ultrasonographic examination of the cervix is highly recommended. This will demonstrate the rate of relaxation of the cervix, the presence of the amniotic sac or parts of the fetus in the cervix or vagina and should be repeated daily according to the following schedule. Rectal palpation can be carried out in combination with ultrasonographic examination.
If there is no relaxation of the cervix at 72–96 hours, repeat rectal examination every 6–12 hours. If there is still no cervix relaxation at 120 hours, see section immediately below. If there is partial relaxation of the cervix at 24–120 hours, administer 50 IU oxytocin SC or IM.
Ruptured Fetal Membranes or 120 hours After Progesterone Drop
The calf should be born within 2 hours after rupture of the fetal membranes. If not, veterinary intervention should take place according to the following schedule. Two hours after rupture of membranes or 120 hours after progesterone drop, collect a blood sample for calcium determination. Store an EDTA and heparin sample for Herpes virus diagnosis (both cells and plasma in freezer after separation). Perform a rectal palpation, transrectal ultrasonographic examination, transrectal massage of the pelvic area with two arms for at least 10 minutes (keep hands gripped together and press with the wrists or the palmar sides of the hands against the pelvic roof and the genital tract). This may stimulate labor activities. Care must be taken not to damage the rectal wall, which may get very edematous. Insert at least one catheter IV. To stimulate further relaxation of the cervix, the parenteral administration of estrogens may be considered. However, no data are available for elephants. Administer 50 IU of oxytocin IM or SC if there is (some) relaxation of the cervix.
Four hours after rupture of membranes or 122 hours after progesterone drop, treat for hypocalcemia if applicable. Perform a rectal palpation, transrectal ultrasonographic examination, and transrectal massage of the pelvic area 50 IU of oxytocin IM or SC. If parts of the fetus are in the pelvic canal, the dose may be increased to 100 IU IM.
Six hours after rupture of the membranes or 124 hours after progesterone drop, perform a rectal palpation, transrectal ultrasonographic examination, check blood Ca-level again, and perform transrectal massage of the pelvic area. If no progress was made, prepare everything to perform a vaginal vestibulotomy. Administer 100 IU of oxytocin in a 1-hour infusion.
Eight hours after rupture of the membranes or 126 hours after progesterone drop, perform a tectal palpation, transrectal ultrasonographic examination, and transrectal massage of the pelvic area. As long as progress continues, monitor and act as above. If no progress was made, perform a vaginal vestibulotomy.
Post-Partum Care
Fluegger et al.1 report that in 20 out of 23 cases the afterbirth was expelled within 10 hours. In one unpublished case at Rotterdam zoo (2001) a piece of afterbirth was found 6 weeks after a normal parturition. No illness of the cow was seen during this period. In Antwerp a 20-year-old cow produced the first calf of a twin (first calving) after a 91-week gestation period. The second calf was born 113 days after the first calf. Both calves were born dead. The second calf was hardly decomposed. The mother did not show significant symptoms of illness (Meurichy, personal communication).
Vaginal Vestibulotomy in Elephants
Vaginal vestibulotomy10-15 is a surgical procedure, in which the vertical part of the urogenital tract (vestibulum vaginae) is exposed by a percutaneous approach. Vaginal vestibulotomy is indicated if there is no progress in calving despite treatment according to the guidelines for veterinary assistance around parturition. Vestibulotomy is contraindicated if there are no fetal parts in the birth canal, confirmed by ultrasonography and rectal palpation.
Protocol of the Procedure
Don’t sedate the cow if not strictly necessary, as you will need the straining support during the extraction. If the animal is non-handleable and restraint in a chute is not an option, use a reversible sedation (xylazine/atipamezole) and antagonize the sedation as soon as the elephant is chained on four legs. Behavior may have changed during this phase of the calving, most likely in favor of the veterinary intervention (i.e., the animal is probably more interested in getting the calf out than in attacking the keepers around her). However, there is not sufficient information about this point. The situation has to be evaluated for each animal.
Use local anesthesia only. After cutting through the skin, place a flexible plastic tube (5–10 cm diameter or a rumen tube) retrograde into the vestibulum vaginae to locate the incision site. To facilitate orientation, a 10 × 2 cm window should be made in the tip of the tube, 3 cm from the end. This window can be palpated transcutaneously and facilitate a quick perforation incision.
Use normal calving chains. Don’t use more than three people per leg. An extra ring in the floor between the hind legs should be present in any calving box for elephants. A pulley can be attached to it in order to provide optimal conditions for pulling the calf out (in a ventral direction). If not present, one should consider to use a steel bar fixed to the walls behind the cow. A pulley can be attached to this bar. When the calf is in posterior position and can’t pass through the pelvic girdle, try to rotate the calf (90° longitudinal axis) during extraction. In cattle, active rotation is the normal way for a live calf to pass its pelvis through the pelvic canal of the mother. When the calf is dead, the absence of this phenomenon might be responsible for stagnation of the calving process. In the experience of one of the authors (Rotterdam Zoo, 1993) it was only possible to remove a dead calf in posterior position by vaginal vestibulotomy after 90° rotation of the calf.
Don’t hesitate to push the calf in the direction of the uterus; it might return in a better position. During this action, both legs and the trunk or tail should be well connected to the chains. Refill the genital tract with at least 5 L of “artificial embryonic fluid” by using a pump (aquarium-type). Don’t pull at both legs in one direction at the same time. You must cross the chains and pull alternatively on each leg. It is more likely that each leg will pass the pelvic canal bit by bit, rather than that the two legs can pass simultaneously. When the calf is out, flush the uterus with cold water until the placenta is expelled. Give 50 IU of oxytocin IV. At this time (if needed) the cow can be given a xylazine sedation (if sedated and reversed previously, use azaperone).
Place a long balloon catheter in the urinary bladder. Cut it to the proper length (i.e., just below the wound). Use acrylic glue or suture it in place. Close the vaginal vestibulum wall in two layers with PDS or Vicryl.
Reverse the sedation, wash your hands, clean up the mess, and have a beer. But don’t close the skin. Forget everything you learned about surgery. Whatever fancy suture technique you may use, the skin wound will open again. Don’t think that your special suture will work, because every possible suture technique has already failed. Even when closing the skin, feces will still contaminate the wound, because the skin property does not allow a watertight wound closure; suturing the skin has proven to result in a permanent fistula.10,11 You may hope that by leaving the skin wound open, the vestibulum sutures may be exposed to a minimum of tension, giving the wound the chance to heal. By doing so, you might be the first veterinarian performing this surgery without leaving the animal with a fistula. Give antibiotics for at least 7 days.
If you were not able to remove the calf, do not try to perform a caesarean unless you are absolutely sure that the cow will not survive conservative treatment. All cesareans performed to date have resulted in the death of the mother. If you see yourself facing the critical situation that the calf cannot be removed through the wound and fetotomy is either no option or it has failed, the first advice is to leave the wound of the vestibulotomy open completely and see what happens during the days to come. Flush the uterus frequently with large amounts of water with a disinfectant like Betadine iodine to prevent the occurrence of Bandl’s rings that may cause necrosis of the uterus.16 An antibiotic treatment is recommended.
Literature Cited
1. Flügger M, Göritz F, Hermes R, Isenbügel E, Klarenbeek A, Schaftenaar W, et al. Evaluation of physiological data and veterinary medical experiences in 31 Asian elephant (Elephas maximus) births in six European zoos. In: Proceedings of the 40th International Symposium of Zoo and Wildlife Animal Medicine. Rotterdam, Netherlands, 2001.
2. Hermes R, Olson D, Göritz F, Brown JL, Schmitt DL, Hagan D, et al. Ultrasonography of the estrous cycle in female African elephants (Loxodonta africana). Zoo Biol. 2000;19:369–382.
3. Hildebrandt Th.B, Göritz F, Pratt NC, Brown JL, Montali RJ, Schmitt DL, et al. Ultrasonography of the urogenital tract in elephants (Loxodonta africana and Elephas maximus): an important tool for assessing female reproductive function. Zoo Biol. 2000;19:321–332.
4. Olsen JH, Chen CL, Boules MM, Morris LS, Coville BR. Determination of reproductive cyclicity and pregnancy in Asian elephants (Elephas maximus) by rapid radioimmunoassay of serum progesterone. J Zoo Wildl Med. 1994;25:349–354.
5. Hodges JK, McNeilly AS, Hess DL. Circulating hormones during pregnancy in the Asian and African elephants: a diagnostic test based on the measurement of prolactin. Int Zoo Yearbook. 1987:26:285–289.
6. Brown JL, Schmitt DL, Bellem A, Graham LH, Lehnhardt J. Hormone secretion in the Asian elephant (Elephas maximus): characterization of ovulatory and anovulatory luteinizing hormone surges. Biol Reprod. 1999;61:1294–1299.
7. Carden M, Schmitt D, Tomasi T, Bradford J, Moll D, Brown J. Utility of serum progesterone and prolactin analysis for assessing reproductive status in the Asian elephant (Elephas maximus) Anim Repro Sci. 1998;53:133–142.
8. Schmidt MJ. Calving elephants (normal). In: Fowler ME, Miller RE, eds. Zoo and Wild Animal Medicine: Current Therapy 4. Philadelphia, PA: W.B. Saunders Company; 1999:521–522.
9. Brown JL, Lehnardt J. Serum and urinary hormones during pregnancy and the peri- and post-partem period in an Asian elephant (Elephas maximus). Zoo Biol. 1995;14:555–564.
10. Schaftenaar W. Vaginal vestibulotomy in an Asian elephant (Elephas maximus). In: Proceedings of the American Association of Zoo Veterinarians. 1996:434–439.
11. Dittrich L. Sensationell: operation rettete elephantin Jenny. Der Zoofreund. 1985;56:2–6.
12. Merkt H, Ahlers D, Bader H, Brandt H-P, Böer M, Dittrich L. Der Dammschnitt, eine geburtshilfliche interventionsmöglichkeit bei der elefantenkuh. Dtsch Tierärztl Wschr. 1985;92:428–432.
13. Merkt H, Ahlers D, Bader H, Brandt H-P, Böer M, Dittrich L. Bildbericht über den Auszug eines toten elefantenfetus (Elephas maximus) am 645. Tag p.c. und 65 Stunden nach Geburtsbeginn via Dammschnitt (Vorläufige Mitteilung). Prakt Tierarzt. 1985;5:377–378.
14. Merkt H, Ahlers D, Bader H, Brandt H-P, Böer M, Dittrich L. Nachbehandlung und Heilungsverlauf bei einer Elefantenkuh nach Geburtshilfe durch Dammschnitt. Berl Münch Tierärztl Wschr. 1986;99:329–333.
15. Strauss G, Czupalla O, Lange A, Hildebrandt T. Der Dammschnitt als Geburtshilfliche Massnahme bei einem Asiatischen Elefanten. Milu. 1998;9:483–495.
16. Foerner JJ. Dystocia in the elephant. In: Fowler ME, Miller RE, eds. Zoo and Wild Animal Medicine: Current Therapy 4. Philadelphia, PA: W.B. Saunders Company; 1999:522–525.