Abstract
In exotic species, replacement of vital fluids is often impossible because of limited resources (lack of donor animals and small sizes of donor animals) available to practitioners. Oxygen therapeutics are a great potential alternative because of availability, compatibility, and safety. Few studies have been completed in non-domestic species.1,6,8 Anecdotally, Oxyglobin® has been used in multiple zoo animals including reptiles and large felids. The purpose of this study was to examine the effects of Oxyglobin® (a polymerized bovine hemoglobin in a modified lactated Ringer’s solution) in rainbow trout in order to ascertain if product use is safe, effective, and feasible in a piscine species.
Sixteen apparently healthy, 3.5-year-old, hatchery-raised rainbow trout (Oncorhynchus mykiss) were anesthetized with 75 ppm tricaine methane sulfonate. Animals were tagged with Floy® tags (Floy Tag and Mfg, Inc., Seattle, WA) for identification. A 2.5 ml/kg single IV bolus of Oxyglobin® (Biopure Corporation, Cambridge, MA) was administered to 14 of the fish after a nearly equal volume of blood was drawn for analysis. Blood analysis was performed pre-treatment, 2 hours post-treatment, and 24 hours post-treatment. The remaining two animals underwent similar anesthesia and phlebotomy events. Three animals were necropsied 24 hours post-treatment. Three animals had blood analysis performed 1 week after treatment and were necropsied.
Recommended doses for mammals are 10–30 ml/kg as an IV bolus or at a rate of up to 10 ml/kg/h; however, due to the nature of the species tested, bolus administration was necessary and injection volumes greater than 2.5 ml/kg could not be reliably delivered. Physical changes were noted, blood parameters evaluated, and select necropsies were performed.
Clinically observable changes in treated animals included transient lethargy, tissue discoloration and, in rare cases, ocular lens changes. Changes in tissue color were consistent with the changes seen in dogs and cats that are administered Oxyglobin®. All animals returned to normal activity and behavior by the third day after treatment. Animals that were not treated and had repeat capture and phlebotomy events did not exhibit lethargy or tissue discoloration.
Blood parameters were evaluated using the i-STAT chemical analyzer (Heska Corporation, Fort Collins, CO) for its ease and reasonable accuracy.4 The EC7+ cartridge was utilized, which provides the following parameters: Na, K, TCO2, iCa, Hct, Hb, pH, PCO2, PO2 HCO3 BEecf, and SO2. Hematocrit and total protein were evaluated by standard capillary tube methods. Hemoglobin was evaluated with a hemoglobinometer (Buffalo Medical Specialties MFG, Inc., Buffalo, NY) by colorimetry. Three animals were randomly selected to run full CBCs and serum chemistries at IDEXX Laboratories (Elmhurst, IL); these values were consistent with overlapping i-STAT values. Blood values were compared to published normal values.2
Oxyglobin® administration results in a color change of serum and urine (similar to hemolysis), therefore producing interference in laboratory testing, which commonly uses colorimetric methods. Studies have shown that there is a maximum allowable concentration of HBOC (hemoglobin-based oxygen carrier) that can still be run on select analyzers.9 Although the i-STAT was not a part of these studies, it can be reasonably assumed that it would act similarly for many tests (but not all), since the methodology is similar. Many analyzers required 50 g/dl (range 4–50 g/dl) of HBOC in the blood before causing interference. Using filtration, other wavelengths in the machine, and, obviously, methods other than colorimetry can circumvent some colorimetric interference.
Determining oxygen-carrying capacity relies on evaluating the ratio of hemoglobin to hematocrit (1:3). Most chemistry machines calculate the hemoglobin from the hematocrit, thereby reducing the accuracy with which the clinician can assess oxygenation. The HemoCue (HemoCue, Inc., Mission Viejo, CA) is a hemoglobinometer that has been shown5 to accurately read hemoglobin in serum that has been mixed with an HBOC. Using this machine can help to assess the oxygen-carrying capacity in treated animals. Unfortunately, this tool was not available to the authors at the time of this study.
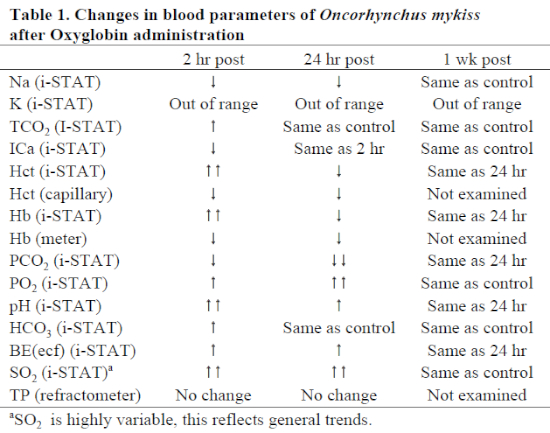
Blood at 2 and 24 hours post-administration had dark red, translucent serum. Blood drawn on three specimens at 1 week post-administration was normal in appearance. There were general trend differences between treatments for various parameters (Table 1); however, none of these values strayed from outside the normal ranges. Of note, pH increased while PCO2 decreased. PO2 and SO2 were widely variable, possibly dependent on whether arterial or venous blood was drawn (both vessels are in proximity to one another). Overall, in treated animals, oxygen saturation was remarkably higher than in either non-treated animals or in pre-treatment samples. Hematocrit as measured by capillary tube and centrifugation was decreased due to blood loss and hemodilution byproduct; the i-STAT Hct results were calculated values and were likely artificially increased due to serum color change. Hemoglobin values (by either handheld colorimetry or i-STAT) were unreliable using the techniques in this study due to color change. Other values seemed reliable and consistent with control animals and commercial laboratory results.
Necropsy at 24 hours post-administration revealed local venipuncture site hemorrhage and tissue discoloration; there were no relevant histopathologic lesions. Necropsy at 7 days showed venipuncture site hemorrhage (repeat phlebotomy) and no relevant histopathologic lesions other than unilateral choroidal edema in one animal.
Pharmacokinetic data (Freedom of Information Summary, NADA 141-067) show that duration of action increases with increasing dose. In those studies, a dose of 10 ml/kg provided a 1 g/dl plasma hemoglobin level for 11–23 hours. In our study, a volume greater than 2.5 ml/kg of Oxyglobin® could not be reliably administered, and extravasation was often a consequence at higher volumes. This may be eliminated by placement of a catheter or slower administration of the product, though the logistics of anesthesia and handling aquatic animals may preclude the ability to safely do this; therefore, multiple doses may be necessary.
One potential problem in the use of HBOCs is vasoconstriction (HBOCs bind nitric oxide selectively) and decreased cardiac output (increased plasma volume). This implies that though blood oxygenation is improved, tissue oxygenation may not be realized.7 At low doses of 3 ml/kg of an HBOC for humans (similar to Oxyglobin®), oxygen-carrying capacity was not augmented sufficiently; this coupled with decreased cardiac output was insufficient7 to adequately increase oxygenation for the patients examined.7 This report is contrary to many studies performed. One such study10 performed exchange perfusion (95% replacement) with Oxyglobin® on sheep. The resulting hemoglobin concentration was 6 g/dl, and the animals showed no distress; further, these animals survived long-term with rapid erythropoiesis. Ultimately, additional research needs to be conducted evaluating tissue oxygenation and dosing in various species.
Oxyglobin® is stable (unopened) at room temperature for 3 years, is readily available, and is promised to be sold in volumes of 60 cc. The information provided here indicates that it is a sensible product to use in fish. Preliminary results imply that blood oxygenation is improved with no deleterious peripheral effects. Tissue oxygenation capacity is unknown, but based on physiologic changes in the muscular tissue, perfusion of blood with Oxyglobin® appears to be significant.
For future studies, use of the HemoCue, measurement of oncotic pressure, pharmacokinetics, and muscle tissue oxygenation could be pursued to evaluate the effects of Oxyglobin® more thoroughly in lower vertebrate species.
Acknowledgments
Special thanks to Cindy Krauss, William Hana, Stacy Schultz, Joel Pond CVT, Robert VanValkenburg, and Stacey Wozniak. Thanks to Michael Kinsel, DVM, DACVP and Karen Lemberger, DVM (Zoo Pathology Program, University of Illinois) for gross and histopathologic analysis.
Literature Cited
1. Abou-Madi, N., Garner, M., Kollias, G.V., Moon, P., Rentko, V.T. 2001. The use of Oxyglobin in birds: preliminary pharmacokinetics and effects on selected blood parameters and tissues. Proceedings of the AAZV. Pp. 77–79.
2. Bowser, P.R. 1993. Clinical pathology of salmonid fishes. In: Stoskopf, M.K. (ed.). Fish Medicine. W.B. Saunders Co., Philadelphia, PA. Pp. 327–332.
3. Callas, D.D., Clark, T.L., Moreira, P.L., Lansden, C., Gawryl, M.S., Kahn, S., Bermes, E.W. 1997. In vitro effects of a novel hemoglobin-based oxygen carrier on routine chemistry, therapeutic drug, coagulation, hematology and blood bank assays. Clin Chem. 43(9): 1744–1748.
4. Grosenbaugh D.A., Gadawski J.E., Muir W.W. 1998. Evaluation of a portable clinical analyzer in a veterinary hospital setting. J Am Vet Med Assoc. 213(5): 691–694.
5. Jahr J.S., Lurie F., Driessen B., Davis J.A., Gosselin R., Gunther R.A. 2002. The HemoCue®, a point of care B-hemoglobin photometer, measures hemoglobin concentrations accurately when mixed in vitro with canine plasma and three hemoglobin-based oxygen carriers (HBOC). Can J Anaesth. 49(3): 243–248.
6. Lichtenberger, M., Rosenthal, K., Brue, R., Kirby, R. 2001. Administration of Oxyglobin and 6% hetastarch after acute blood loss in psittacines birds. AAV Proceedings. Pp: 15–18.
7. Meyer, R. 2001. Current topics in fluid therapy: Oxyglobin. In: Gleed, R.D. and Ludders, J.W. (eds.). Recent Advances in Veterinary Anesthesia and Analgesia: Companion Animals. International Veterinary Information Service, Ithaca, NY.
8. Orcutt, C. 2000. Oxyglobin administration for the treatment of anemia in ferrets. Exotic DVM. 2(3): 44–46.
9. Sarkozi, L., Jacobs, E., Clark, T., Gawryl, M.S., Simson, E. 1997. Effect of hemoglobin-based oxygen carrier-201 on common chemistry laboratory procedures. Clin Chem. 43(9): 1792–1794.
10. Vlahakes, G.J., Lee, R., Jacobs, E.E., LaRaia, P.J., Austen, W.G. 1990. Hemodynamic effects and oxygen transport properties of a new blood substitute in a model of massive blood replacement. J Thorac Cardiovasc Surg. 100: 379–388.