H. Poulet, DVM; S. Brunet, PhD, D.JAS DVM; G. Chappuis, DVM
Feline calicivirus (FCV) is a major pathogen of the domestic cat causing upper-respiratory tract infection, oral vesicular disease and chronic stomatitis. FCV is characterized by a high level of antigenic and pathogenic variation. Some new hypervirulent variants have recently emerged in North America causing a high rate of mortality, even in vaccinated cats (Pedersen et al., 2000; Shorr-Evans et al., 2003).
In spite of widespread vaccination, prevalence of FCV infection is still very high (Harbour et al., 1991; Binns et al., 2000), and current vaccines which reduce clinical signs and virus shedding may not protect against infection. Efficient vaccines able to reduce viral excretion would be expected to aid in decreasing FCV prevalence in the cat population. Unfortunately, attempts to improve the efficacy of FCV vaccines have failed so far. Second generation DNA or vectored vaccines have been shown to provide only partial clinical and virological protection (McCabe et al., 2002; Sommerville et al., 2002; Schwantes et al., 2003). Mucosal immunization using feline herpesvirus vector containing the capsid gene of FCV failed to improve vaccine efficacy (Yokoyama et al., 1998).
Another challenge for the development of efficacious FCV vaccines is related to the high antigenic variability of the virus (Baulch-Brown et al., 1999). In natural conditions, vaccinated animals are exposed to heterologous strains, and it is likely that current vaccines afford only protection against a fraction of the circulating FCV field isolates (Lauritzen et al., 2000; Pedersen et al., 2000). Increasing heterologous protection against antigenically distant strains could therefore be a way to improve the efficacy of current vaccines.
Several studies have suggested that modified live strains from attenuated vaccines might contribute to the emergence of new isolates in the feline population (Dawson et al., 1993; Pedersen and Floyd Hawkins, 1995; Radford et al., 1997; Radford et al., 2001). This may be related to the residual virulence of attenuated strains and the high rate of mutation of FCV.
Is there a need for vaccine modification (Baulch-Brown et al., 1997) ? Existing scientific data suggest that there is need for new vaccines:
 Using recent FCV isolates as antigens, with a broader range of protection
Using recent FCV isolates as antigens, with a broader range of protection
 Which do not contain attenuated live FCV strains, because of potential safety issues
Which do not contain attenuated live FCV strains, because of potential safety issues
 With better efficacy (the ultimate goal would be to prevent infection)
With better efficacy (the ultimate goal would be to prevent infection)
SELECTING FCV STRAINS
Recent FCV isolates were included in extensive cross-neutralization studies (Figure 1). Antigenic dominance and relatedness between strains was calculated according to the method described by others (Chappuis and Stellmann, 1974). This study confirmed that current vaccine strains (FCV255 and FCV F9) are not well adapted to current field FCV isolates. Their antigenic dominance (ability to induce cross-neutralizing antibodies) was the lowest of the tested strains. Interestingly, isolates from cats with chronic infection had a lower antigenic relatedness than the acute infection ones (Poulet et al., 2000). Both observations are probably the result of the antigenic shift of FCV driven by immune pressure induced by current vaccine strains.
| Figure 1. | 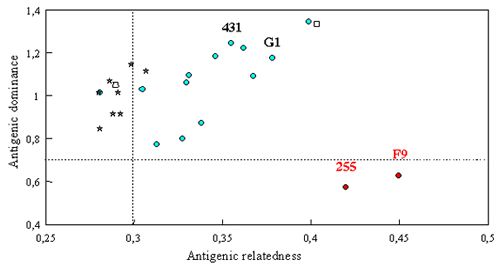
Cross-neutralization between recent isolates and vaccine strains. Isolates from cats with chronic infection (stars) have a lower antigenic relatedness than cats with acute infection (circles). History of two isolates (unfilled squares) was unknown. Vaccine strains FCVF9 and FCV255 have a low antigenic dominance. |
|
| |
The antigenic diversity within FCV isolates was confirmed by immunofluorescence analysis using a panel of monoclonal antibodies mapped on the main epitopes of the capsid protein. All isolates were different from one another.
Two recent isolates FCV431 and FCV G1 were identified as immunodominant strains. FCV431 and FCVG1 specific antisera from infected cats could neutralize more than 90% of a large panel of isolates from different countries.
Some authors have suggested that vaccines should contain more than one vaccine strain (Dawson et al., 1993; Baulch-Brown et al., 1997; Schneider and Truyen, 1998). Therefore, the advantage of combining two strains was evaluated. Cats were immunised with live FCV431, live FCV G1 or the combination of both strains, via the sub-cutaneous route. The animals were then challenged with antigenically distant virulent isolates (FCV220 and FCV393) via the oro-nasal route. FCV220 and FCV393 induced severe clinical signs in control cats typical of FCV infection. Immunisation with the combination of FCV431 and FCVG1 induced higher neutralising antibody titres against FCV220 and FCV393 strains on average. Protection was observed in all groups, however combination of the two strains resulted in a better clinical protection and reduction of virus shedding after heterologous challenge (Figure 2). These results indicated that vaccines combining antigens from different FCV strains may induce a broader heterologous protection.
Sequencing of the entire capsid gene showed that FCVG1 and FCV431 shared the same degree of distance as any pair of strains from the presented panel. These two strains were considered as distinct strains, since they displayed 29% of nucleotides distance in the hypervariable (HVR) E region of the capsid protein. Distance value between region E sequences from epidemiologically unrelated isolates ranges from 21 to 38% (Radford et al., 1997).
| Figure 2. | 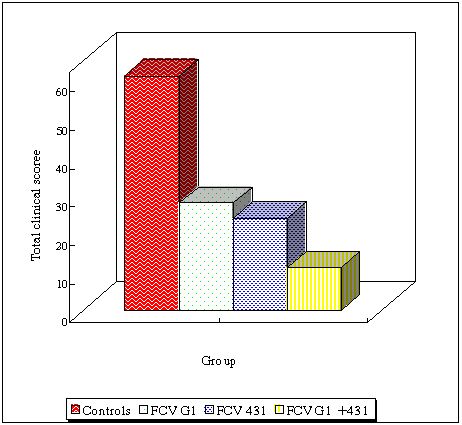
Total clinical score in controls, cats immunised with FCVG1 only, with FCV431 only or with the combination of FCV431 and FCVG1. This graph compiles results from FCV220 and FCV393 challenges. |
|
| |
SELECTING VACCINE TECHNOLOGY
After selection of FCV strains, an adapted technology was used to develop a new vaccine. Since the tested recombinant technologies did not provide any significant advantage in this particular context (Yokoyama et al., 1998; McCabe et al., 2002; Sommerville et al., 2002; Schwantes et al., 2003), it was decided to develop an inactivated vaccine. Since adjuvants compromise local tolerance and may increase the risk of serious adverse reactions at injection site (Gobar and Kass, 2002), the challenge was to develop an inactivated FCV antigen which was effective without adjuvant.
A combined vaccine against viral rhinotracheitis (FHV), calicivirosis, infectious panleucopenia (FPV), chlamydiosis and feline leukaemia was developed. The calicivirus component consisted of a combination of two purified inactivated FCV antigens derived from FCV431 and FCVG1 strains. The efficacy of this vaccine was compared with that of a reference vaccine containing the FCV F9 attenuated strain. Four groups of 6 cats were vaccinated with combined vaccines containing the new FCV vaccine (groups 0RM725 5T24, 5M18 and 5U25) or with a reference vaccine with FCV F9 attenuated strain. A fifth group of 6 kittens was not vaccinated (control group). After challenge with virulent FCV255 via the oro-nasal route, the cats were examined daily for rectal temperature and calicivirosis clinical signs (oro-nasal ulcers, ocular and nasal discharge, lethargy...), and were weighed at regular intervals. Challenge induced hyperthermia, weight loss and oro-nasal ulcers in controls. Clinical signs were significantly reduced in vaccinates. The new FCV vaccine performed better than the attenuated FCV F9 strain (Figure 3). Vaccination induced also a strong reduction in viral excretion after challenge.
| Figure 3. | 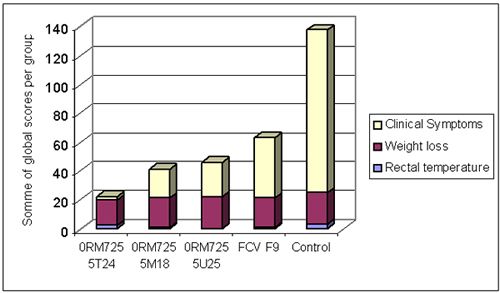
Total clinical score after FCV255 challenge. Clinical signs consisted mainly of hyperthermia, weight loss and oronasal ulcers. The first 3 groups were vaccinated with the new FCV vaccine (at different doses), the fourth group with a reference FCV F9 attenuated strain and the fifth group was not vaccinated (controls). |
|
| |
This first trial showed that the new FCV inactivated non-adjuvanted antigen was effective against a virulent heterologous FCV challenge.
The duration of immunity induced by this new FCV vaccine was shown to last for at least one year. Cats challenged one year after primo-vaccination were significantly protected against virulent challenge.
Efficacy was also demonstrated against other heterologous challenge strains including an antigenically very distant one.
The efficacy of this new FCV vaccine was also demonstrated in young kittens with maternally derived antibodies. MDA against FCV persist in kittens older than 8 weeks and may be a concern for vaccine efficacy (Dawson et al., 2001). Vaccination of 7.4 to 10.6 week old kittens induced a seroconversion in more than 92% of the cats.
CONCLUSION
In conclusion, a new FCV vaccine was developed to improve:
 Efficacy: existing vaccines are not well adapted to current FCV isolates because of an antigenic shift in the virus population
Efficacy: existing vaccines are not well adapted to current FCV isolates because of an antigenic shift in the virus population
 Safety: a non-adjuvanted inactivated FCV vaccine overcomes safety issues raised with modified live or inactivated adjuvanted vaccines
Safety: a non-adjuvanted inactivated FCV vaccine overcomes safety issues raised with modified live or inactivated adjuvanted vaccines
The efficacy of this new vaccine was extensively studied and characterized by:
 A strong heterologous efficacy with significant reduction of clinical signs and excretion
A strong heterologous efficacy with significant reduction of clinical signs and excretion
 A duration of immunity of one year demonstrated by serology and challenge
A duration of immunity of one year demonstrated by serology and challenge
 Its compatibility with other feline vaccines (viral rhinotracheitis, panleucopenia and chlamydiosis)
Its compatibility with other feline vaccines (viral rhinotracheitis, panleucopenia and chlamydiosis)
 Its efficacy in presence of maternally derived FCV antibodies
Its efficacy in presence of maternally derived FCV antibodies
References
1. Baulch-Brown, C., Love, D.N., Meanger, J., 1997. Feline calicivirus: a need for vaccine modification ? Aust. Vet. J. 75(3), 209-213.
2. Baulch-Brown, C., Love, D.N., Meanger, J., 1999. Sequence variation within the capsid protein of Australian isolates of feline calicivirus. Vet. Microbiol. 68, 107-117.
3. Binns, S.H., Dawson, S., Speakman, A.J., Cuevas, L.E., Hart, C.A., Gaskell, C.J., Morgan, K.L., Gaskell, R.M., 2000. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. Journal of Feline Medicine and Surgery 2, 123-133.
4. Chappuis, G., Stellmann, C., 1974. Etude sérologique des picornavirus félins: parenté-dominance des souches. Bull.Soc.Sci.Vet et Med comparée, 76(4), 289-299.
5. Dawson, S., McArdle, F., Bennett, D., Carter, M., Bennett, M., Ryvar, R., Gaskell, R.M., 1993. Investigation of vaccine reactions and breakdowns after feline calicivirus vaccination. Vet. Rec. 132, 346-350.
6. Dawson, S., McArdle, F., Bennett, M., Carter, M., Milton, I.P., Turner, P., Meanger, J., Gaskell, R.M., 1993. Typing of feline calicivirus isolates from different clinical groups by virus neutralisation tests. Vet. Rec. 133, 13-17.
7. Harbour, D.A., Howard, P.E., Gaskell, R.M.,1991. Isolation of feline calicivirus and feline herpesvirus from domestic cats 1980 to 1989. Vet. Rec. 128, 77-80.
8. Lauritzen, A., Jarrett, O., Sabara, M., 1997. Serological analysis of feline calicivirus isolates from the United States and United-Kingdom. Vet. Microbiol. 56, 55-63.
9. McCabe, V.J., Tarpey, I., Spibey, N., 2002. Vaccination of cats with an attenuated recombinant myxoma virus expressing feline calicivirus capsid protein. Vaccine 20, 2454-2462.
10. Pedersen, N.C., Elliott, J.B., Glasgow, A., Poland, A., Keel, K., 2000. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 73, 281-300.
11. Poulet, H., Brunet, S., Soulier, M., Leroy, V., Goutebroze, S., Chappuis, G., 2000. Comparison between acute oral/respiratory and chronic stomatitis/gingivitis isolates of feline calicivirus: pathogenicity, antigenic profile and cross-neutralisation studies. Arch. Virol. 145, 243-261.
12. Radford, A.D., Bennett, M., McArdle, F., Dawson, S., Turner, P.C., Glenn, M.A., Gaskell, R.M.,1997. The use of sequence analysis of a feline calicivirus (FCV) hypervariable region in the epidemiological investigation of FCV related disease and vaccine failures. Vaccine 15(12/13), 1451-1458.
13. Radford, A.D., Dawson, S., Wharmby, C., Ryvar, R., Gaskell, R.M., 2000. Comparison of serological and sequence-based methods for typing feline calicivirus isolates from vaccine failures. Vet. Rec. 146, 117-123.
14. Radford, A.D., Dawson, S., Ryvar, R., Coyne, K., Johnson, D.R., Cox, M.B., Acke, E.F.J., Addie, D.D., Gaskell, R.M., 2003. High genetic diversity of the immunodominant region of the feline calicivirus capsid gene in endemically infected cat colonies. Virus Genes 27(2), 145-155.
15. Schorr-Evans, E.M., Poland, A., Johnson, W.E., Pedersen, N.C., 2003. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. Journal of Feline Medicine and Surgery 5, 217-226.
16. Schwantes A., Truyen U., Weikel J., Weiss C., Löchelt M., 2003. Application of chimeric Feline Foamy Virus based retroviral vectors for the induction of antiviral immunity in cats. J. Virol. 77(14), 7830-7842.
17. Sommerville, L.M., Radford, A.D., Glenn, M., Dawson, S., Gaskell, C.J., Kelly, D.F., Cripps, P.J., Porter, C.J., Gaskell, R.M., 2002. DNA vaccination against feline calicivirus infection using a plasmid encoding the mature capsid protein. Vaccine 20, 1787-1796.
18. Yokoyama, N., Fujita, K., Damiani, A., Sato, E., Kurosawa, K., Miyazawa, T., Ishiguro, S., Mochizuki, M., Maeda, K., Mikami, T., 1998. Further development of a recombinant Feline Herpesvirus type 1 vector expressing Feline Calicivirus immunogenic antigen. J. Vet. Med. Sci. 60(6), 7