Development of a Xenogeneic DNA Vaccine Program for Canine Malignant Melanoma at the Animal Medical Center
Philip J. Bergman, DVM, MS, PhD DACVIM (Oncology)
Head, Donaldson-Atwood Cancer Clinic & Flaherty Comparative Oncology Laboratory Adjunct Associate Faculty Member, Memorial-Sloan-Kettering The Animal Medical Center
NYC, NY, USA
INTRODUCTION
Canine malignant melanoma (CMM) of the oral cavity, nail bed, foot pad and mucocutaneous junction is a spontaneously occurring, highly aggressive and frequently metastatic neoplasm. CMM is a relatively common diagnosis representing ~ 4% of all canine tumors and it is the most common oral tumor in the dog. CMM and advanced human melanoma (HM) are diseases that are initially treated with aggressive local therapies including surgery and/or fractionated radiation therapy; however, systemic metastatic disease is a common sequela. Also, CMM and HM are chemoresistant neoplasms. Based on these similarities, CMM is a good clinical model for evaluating new treatments for advanced HM.
Canine patients with advanced disease (WHO stage II, III or IV) have a reported median survival time of 1-5 months with aggressive local excision. Dogs with > 90% stage I melanoma treated with hypofractionated radiation therapy and chemotherapy have a reported median survival time of one year (Freeman et al.). Human patients with deep AJCC stage II or stage III disease (locally advanced or regional lymph node involvement) have at least a 50% chance of recurrence after surgical resection; patients with stage IV melanoma (distant metastases) have a median survival of less than ten months and most of these patients eventually die of melanoma. Standard systemic therapy is dacarbazine chemotherapy in HM, and carboplatin chemotherapy in CMM. Unfortunately, response rates to chemotherapy in humans or dogs with advanced melanoma range from 8-28% with little evidence that treatment improves survival. It is easily evident that new approaches to this disease are desperately needed.
Active immunotherapy in the form of vaccines represents one potential therapeutic strategy for melanoma. The advent of DNA vaccination circumvents some of the previously encountered hurdles in vaccine development. DNA is relatively inexpensive and simple to purify in large quantity. The antigen of interest is cloned into a bacterial expression plasmid with a constitutively active promoter. The plasmid is introduced into the skin or muscle with an intradermal or intramuscular injection. Once in the skin or muscle, professional antigen presenting cells, particularly dendritic cells, are able to present the transcribed and translated antigen in the proper context of major histocompatibility complex and costimulatory molecules. The bacterial and plasmid DNA itself contains immunostimulatory sequences that may act as a potent immunological adjuvant in the immune response. In clinical trials for infectious disease, DNA immunization has been shown to be safe and effective in inducing immune responses to malaria and human immunodeficiency virus. Although DNA vaccines have induced immune responses to viral proteins, vaccinating against tissue specific self-proteins on cancer cells is clearly a more difficult problem. One way to induce immunity against a tissue specific differentiation antigen on cancer cells is to vaccinate with xenogeneic antigen or DNA that is homologous to the cancer antigen. It has been shown that vaccination of mice with DNA encoding cancer differentiation antigens is ineffective when self-DNA is used, but tumor immunity can be induced by orthologous DNA from another species.
We have chosen to target defined melanoma differentiation antigens of the tyrosinase family. Tyrosinase is a melanosomal glycoprotein, essential in melanin synthesis. The full length human tyrosinase gene was shown to consist of five exons and was localized to chromosome 11q14-q21. Immunization with xenogeneic human DNA encoding tyrosinase family proteins induced antibodies and cytotoxic T cells against syngeneic B16 melanoma cells in C57BL/6 mice, but immunization with mouse tyrosinase related DNA did not induce detectable immunity. In particular, xenogeneic DNA vaccination induced tumor protection from syngeneic melanoma challenge and autoimmune hypopigmentation. Thus, xenogeneic DNA vaccination could break tolerance against a self tumor differentiation antigen, inducing antibody, T-cell and anti-tumor responses.
EXPERIMENTAL DESIGN
From April 2000 to present, approximately 60 dogs with previously histologically confirmed spontaneous malignant melanoma were treated with xenogeneic DNA vaccinations. Pre-trial evaluation included complete physical examination, a complete blood count and platelet count, serum chemistry profile, urinalysis, lactate dehydrogenase, anti-nuclear antibody, and three-dimensional measurements of the primary tumor if present (or maximal tumor size from medical records if patient was treated prior to pre-trial considerations). For evaluation of metastatic disease, 3-view radiographs of the thorax were obtained and regional lymph nodes were evaluated with fine needle aspiration/cytology and/or biopsy/histopathology. All dogs were clinically staged according to the WHO staging system of stage II (tumors 2-4 cm. diameter, negative nodes), stage III (tumor > 4 cm. and/or positive nodes) or stage IV (distant metastatic disease). The numbers of previous treatments with surgery, radiation and/or chemotherapy were recorded. Dogs with WHO stage II (high-grade), III or IV histologically confirmed malignant melanoma were allowed entrance onto the study due to the lack of effective available systemic treatments. Additional entry criteria included: an estimated life expectancy of 6 weeks or more, free of clinically detectable brain metastases, no previous therapy (surgery, radiation and/or chemotherapy) for at least 3 weeks and no serious intercurrent medical illnesses. Written consent for entry onto this trial was obtained from each dog's owner prior to entry onto the study; this consent included request for necropsy upon death due to any reason. This study was performed under Animal Medical Center IRB approval.
Cohorts of three dogs each received increasing doses of xenogeneic plasmid DNA encoding either human tyrosinase (huTyr), murine GP75 (muGP75), or murine tyrosinase (muTyr) intramuscularly (100 mcg, 500 mcg and 1500 mcg per dose for each dose level) biweekly for a total of 4 vaccinations in the left caudal thigh with the Biojector 2000 jet delivery device with #3 (intramuscular) Bioject syringes. The Biojector2000 is a carbon dioxide powered jet delivery device which is FDA approved for administration of intramuscular injections and has been used in DNA vaccine clinical investigations. Subjective pain level responses and post-vaccinal presence of a wheal or other reaction were assessed and recorded by the veterinarian administering the DNA vaccination. The dogs did not receive any concomitant treatments during the course of vaccination.
RESULTS
The signalments of dogs on this study have been similar to those in previously reported CMM studies. No toxicity was seen in any dogs receiving the aforementioned vaccines with the exception of minimal to mild pain responses at vaccination and one muGP75 dog experienced mild aural depigmentation. Nine dogs fit the sub-category of stage II-III loco-regionally controlled CMM across the three vaccine studies. The Kaplan-Meier (KM) median survival time (MST) for this sub-population is 589 days as shown in Figure 1, with four dogs censored at 496 (#2), 501 and 517 days due to death from other reasons without any evidence of melanoma at necropsy (3/4) or alive/NED (1/4). The KM MST for all stage II-IV dogs treated with huTyr, muGP75 and muTyr are 389, 153 and 224 days, respectively as shown in Figures 2 and 4. The results from dogs vaccinated with huTyr have been recently published (Bergman et al., Clin Cancer Res 2003, 9:1284-1290).
| 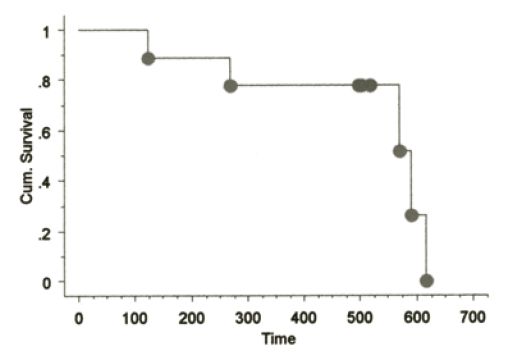
Figure 1. KM Survival curve for nine dogs with minimal residual disease |
|
| |
| 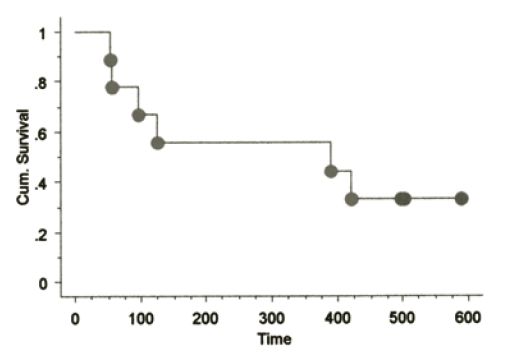
Figure 2. KM Survival curve for nine dogs treated with huTyr |
|
| |
| 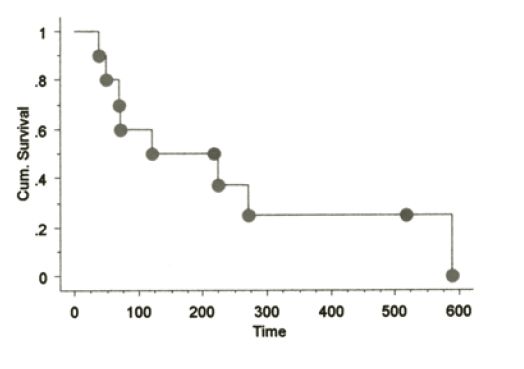
Figure 4. KM Survival curve for dogs treated with muGP75 |
|
| |
CONCLUSIONS
The results of these trials demonstrate that xenogeneic DNA vaccination in CMM is safe, potentially therapeutic, and an attractive candidate for further evaluation in an adjuvant, minimal residual disease setting for CMM. In addition, CMM is a more clinically faithful therapeutic model for HM when compared to more traditional mouse systems as both diseases are chemoresistant, radioresistant, share similar metastatic phenotypes/site selectivity, and occur spontaneously in an outbred, immunocompetent scenario. Similarly, this work shows that veterinary cancer centers and human cancer centers can work productively together to benefit veterinary and human patients afflicted with cancer.
REFERENCES
References are available upon request.