Laboratory of Veterinary Pathology, Graduate School of Agricultural and Life Sciences, The University of Tokyo
Tokyo, Japan
Senile plaque is one of the most characteristic histopathological changes of aged or Alzheimer's disease brain in man and is also detected in the aged brain of other mammalian species including dogs, cats, bears, monkeys, and camels. The morphology of senile plaque is variable among species. In humans, dogs, monkeys and bears, the plaque can be morphologically classified into two types, diffuse and mature, while cats and camels show only diffuse type plaque. Some reports stated that the diffuse plaque was a precursor of the mature type in the human brain, while others proposed that the two types emerged separately. In the case of non-human animals, particularly dogs and monkeys, the two types of senile plaque are also thought to be formed separately. Comparative morphological studies of senile plaque, therefore, would provide new concepts in the field of Alzheimer's disease.
The fractal dimension (FD) is a concept to determine the morphological complexity of an object and has been applied to morphological estimation in both clinical and experimental medicine. However, analysis of senile plaques using FD has not yet been reported.
In the present study, the FD was applied to morphological estimation of animal and human senile plaque using a computer-aided method. The FD of mature plaque (MP) in a 17-year-old dog was significantly higher than that of diffuse plaque (DP) in 11- to 16-year-old dogs (Table 1). In both types of plaque, the FD tended to increase as the plaque size expanded and there was a significant difference between the slope values of the approximate line for diffuse and mature plaque (Fig. 1). In humans, there was also a significant difference in FD value between diffuse and mature plaque (Table 2). No significant differences were observed between the two types of plaque in a bear or a cynomolgus monkey (Table 2). The FD of feline diffuse plaque was significantly lower than that of a camel, bear and monkey (Table 2). These results indicated that the diffuse and mature plaque of the dog might form in a different manner, and similar events may occur in human senile plaque formation. In addition, specific shapes and different FD values of the diffuse plaque among animals suggested that the original conditions for plaque formation would be different.
The three-dimensional (3D) distribution of amyloid beta protein (A beta subtypes (A beta 40 and A beta 42(43)) in canine senile plaques was observed using a confocal laser-scanning microscope. In DP, A beta 42(43) alone was deposited as an uneven nebula-like assembly of fine granules. The border of the A beta 42(43) assembly was unclear and diffusely merged to the surrounding area. MP, on the other hand, showed two patterns of A beta deposition. In some MP, only A beta 40 was deposited as a defined assembly of very short fibrillary structures. Other MP consisted of both A beta 40 and A beta 42(43), and the deposition patterns of the two A beta species were the same as those in single-positive plaques; fine granular with unclear margin for A beta 42(43), and short fibrillary structures for A beta 40.
Additionally, we calculated the FDs of both A beta 40 and A beta 42(43) assemblies, and examined the serial change of FDs in each SP (Fig.2 and Table 3). The FDs of A beta 42(43)-positive DP ranged from 1.05 to 1.27, and those of A beta 40-positive MP ranged from 1.13 to 1.54 in single-positive plaques. In one double-positive MP, FDs ranged from 1.02 to 1.36 for A beta 42(43) and from 1.01 to 1.51 for A beta 40. These results showed that the FD of canine A beta 40 assemblies was higher than that of A beta 42(43) assemblies, and the spatial changes
of FD values for A beta 40 and A beta 42(43) in double-positive plaques were quite different.
The present study indicated that the DP and MP of the dog were formed by different processes, and the same event would occur in human senile plaque formation. In addition, the feline and camel plaques showed specific shapes, and the FD of the feline plaque was different from that of the other animals, indicating the original condition for plaque formation would be different among species. Factors determining the morphology of the senile plaque of each animal species were supposed to be the chemical structure of beta amyloid protein, cerebral microenvironment during plaque formation, and/or metabolic speed or longevity of the species. Further analyses of senile plaque formation from the viewpoint of comparative pathology in addition to using biochemical methods are required, and 3D and fractal analyses would be powerful tools for such investigations.
Table 1. Fractal dimension of canine senile plaques
|
|
Dog
number |
Age |
Fractal dimension
(mean±SD) |
|
Diffuse |
|
|
1.656±0.046* |
|
|
7
11
14
24
34
38 |
16y
15y
16y
15y
11y
14y |
1.686±0.036
1.690±0.024
1.676±0.037
1.646±0.045
1.618±0.040
1.622±0.039 |
|
Mature |
100 |
17y |
1.721±0.048* |
PAM staining. Using x 40 objective lens.
*Significant (p=0.00033) by the Kruskal-Wallis rank test.
Table 2. Fractal dimension of senile plaques in various animal species and in humans
|
Animal |
Plaque type |
Fractal dimension (mean±SD) |
|
Cat |
Diffuse |
1.468±0.051* |
|
Camel |
Diffuse |
1.666±0.025 |
|
Dog |
Diffuse
Mature |
1.656±0.046**
1.721±0.048 |
|
Bear |
Diffuse
Mature |
1.696±0.049
1.721±0.053 |
|
Monkey |
Diffuse
Mature |
1.664±0.040
1.670±0.032 |
|
Human |
Diffuse
Mature |
1.632±0.041***
1.669±0.030 |
PAM staining. Using x 40 objective lens.
* Significant when compared to the FD of camel (p=0.0469), dog (p=0.0005), bear (p=0.0002) and monkey (p=0.0047) diffuse plaques.
** Significant when compared to the FD of canine mature plaques. p=0.0003.
*** Significant when compared to the FD of human mature plaques. p=0.0412.Other combinations were not significant. The Kruskal-Wallis rank test were used.
Table 3. The ratio of the base width and height of FD curve in Fig.2
|
Plaque type |
Aβ species |
h/w a (numberb) |
|
DP |
Aβ 42(43) |
0.14±0.03 (4) |
|
MP |
Aβ 40 |
0.22±0.05 (4) * |
|
MP |
{ Aβ 42(43)
Aβ 40 |
0.16±0.02 (4)
0.22±0.04 (4) * |
a The ratio of the base width (w) and height (h) of FD curve. Average ±S .D.
b The number of SP analyzed.
* Significant (p<0.05) when compared with the value of DP by Student t-test.
Click on image to see a larger view
| 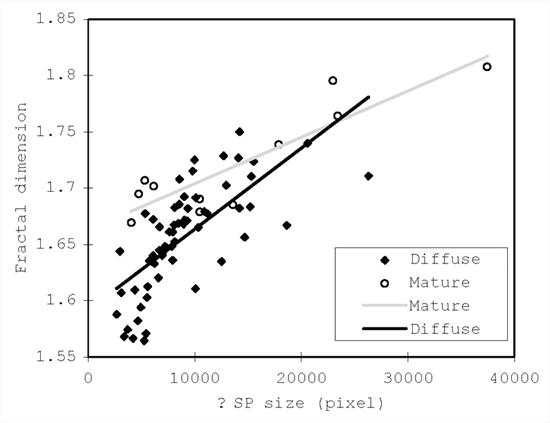
Figure 1. Relationship between the FD and size of canine senile plaques. The black line is for diffuse plaques (y=0.0174x+1.5918 ) and the gray line is for the mature type (y=0.0099x+1.6622). The different slope values of the two lines may indicate different origins and processes of the two types of plaque. |
|
| |
Click on image to see a larger view
| 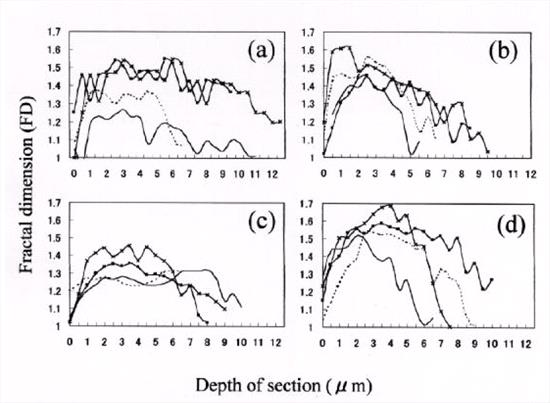
Figure 2. FD of A beta assembly plotted against the depth of the section. Each curve represents one SP. Curves of A beta 42(43) assemblies of DP are trapezoidal shaped, with a broad base and a moderate height (a). Curves of A beta 40 assemblies of single-positive MP are bell-shaped, with a less broad base and a comparatively high height (b). Curves of A beta 42(43) (c) and A beta 40 (d) assemblies of double-positive MP are trapezoidal and bell-shaped, respectively. |
|
| |