Summary
Heartworm is a major potentially life-threatening disease of dogs in Thailand which it is spread through mosquito bites. All dogs living in a heavily populated mosquito area are at risk. It is possible that most dogs in endemic foci could be at risk for heartworm new infection and reinfection every year. Dogs may not show signs of illness until the disease is severe. Through the surveys, the high prevalence of Dirofilaria immitis infection has been evident in the stray and pet dogs. Most infected dogs can be successfully treated for heartworms if the disease is detected early. Also prevention with monthly medication has proven to be highly successful in controlling heartworm infection.
Introduction
Dirofilaria immitis, a mosquito-borne nematode parasite which normally resides in the right ventricle and pulmonary arteries of dogs causing exercise intolerance, coughing, edema, ascites, heart failure or death of the infected dogs. It is widespread and endemic in the tropics, subtropics and temperate zones around the world (Haddock, 1987). The mosquito is the only natural vector of transmission for canine heartworms with approximate 70 species of them being implicated as vectors. The infective stage, L3 developed in the potential vectors which are commonly found in the urban and suburban areas. After dog is infected with D. immitis infective L3, the initial development of parasite takes place at the infection site before migrating to the right heart, reaching maturity, producing their progeny, the so-call microfilaria and circulating into the peripheral blood.
Epidemiological studies of canine heartworm infection and disease transmission in Thailand
Dirofilariasis is a currently high prevalence in dog population but less common in cats. Epidemiological studies on heartworms in Thailand suggested that there were a high incidence of D. immitis among stray and pet dogs. The status of D. immitis infection in stray dogs was evaluated from dogs at animal shelter, the rabies control center, Bangkok Metropolitans. The positive rate of infection of adult worms from necropsies was 24.6-51.0% (Sankavoranond,1981; Sankavoranond and Paiboolratanawong, 2001). In 1992, Nithiuthai and Chungpivat showed a high incidence of microfilariae in peripheral blood of 496 stray dogs with a positive rate of 46.17% as determined by modified knott's concentration and three-line thick smears of the Giemsa's staining and acid phosphatase activity. A similar result (45.67%) of microfilariae was observed from peripheral blood of stray dogs in Chiangmai (Choochote et al., 1987). Assessment of a condition of amicrofilaremic or occult dirofilariasis was determined by necropsies, knott's and antibody-ELISA and antigen-ELISA. The overall occurrence of occult dirofilariasis in stray dogs was 10-72.50% (Techodom et al., 1992 ; Chuanchuen et al., 1993).
Recent studies on the prevalence of D. immitis infection in pet dogs was assessed from 2 diagnostic laboratory services during the year 1999-2002. A total of 83,476 specimens of anticoagulant blood and sera from over 300 private animal clinics and hospitals in Bangkok were determined. The status of heartworm infection was evaluated by the presence of microfilariae using conventional methods of a stained buffy-coat film. Suspected cases were further tested by modified knott's concentration and/or antigen-ELISA kits (SnapR , Idexx, U.S.A. and WitnessR, Agen, Australia). The overall incidence of heartworm infection in pet dogs in Bangkok is shown in figure 1.
Number of cases
| 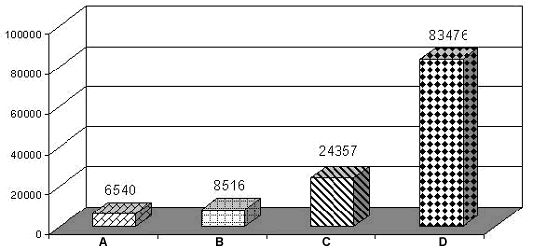
Figure 1. A graph showing number of positive cases of Dirofilaria immitis infection (A-C) comparing with 83,476 total blood samples (D) of pet dogs examined by 3 techniques during 1999-2002. |
|
| |
A = stained buffy-coat
B = stained buffy-coat and knott's concentration
C = stained buffy-coat, knott's concentration and Antigen-ELISA
D = Total blood samples
It is evident that a total of 8,516 positive samples (10.2%) were D. immitis microfilariae. Knott's concentration technique gave a 10.8% better result than stained buffy-coat blood films. Together with amicrofilaremic D. immitis infected cases, the overall prevalence of D. immitis infection in pet dogs was 29.2%. It is proved that the antigen-ELISA detection is superior over conventional methods and gave 30.9% better result than those conventional ones. Hence all conventional methods for circulating microfilaremiae detection is inadequate in comparison to circulatory antigen detection. From this study a total of 235 samples out of 1,112 amicrofilaremic cases showed a positive antigen-ELISA test for heartworm infection. This may be due to a prepatent or developmental period, unisexual or post treatment sterile infections leading to high incidence of amicrofilaremic state. Therefore one should be aware of alternative test for the diagnosis of heartworm infection in endemic area. However such preferable alternative test as circulating antigens detection will give the best result only with the presence of adult female worms. In the present study, the overall prevalence of heartworm infection in pet dogs in Bangkok was as high as 29.2%. While the other study found only 11.78% of microfilaremia in pet dogs in suburban area (Salakij et al., 1999).
It has been well documented that the prevalence of heartworm infection in dogs increased with age. Establishment of adults D. immitis in dogs would be assisted by the long life expectancy of the parasite which would facilitate the transmission. The possible reason for higher prevalence of the infection in older dogs may be due to their longer time of exposure which is an important risk factor in endemic area.
In Thailand the environmental conditions are favorable for the transmission of D. immitis where the parasite becomes available. The density of several species of mosquito vectors is reasonably high throughout the country. The optimal temperature and humidity for development of the infective larvae in mosquitoes are the greatest factors for the establishment of this filarial nematode. Surathint et al.(1985) showed that the infective larvae of D. immitis experimentally developed in 3 species of mosquitoes, Mansonia uniformis, Ma. indiana and M. annulifera while Choochote et al.(1987) were sucessfully infected D. immitis in 4 species of mosquitoes; M. uniformis, Culex quinquefasciatus, Ae. albopictus and Ae. aegypti. In addition, the infective L3 of D. immitis were demonstrated in 5 species of naturally wild caught female mosquitoes, Ma. uniformis, Cx. quinquefasciatus, Cx. tritaeniorhynchus, Cx. gelidus and Ae. aegypti in the rural area of Chiangmai (Choochote et al., 1986 ; Choochote et al., 1992). Such species presumably play an important role as the potential mosquito vectors of canine heartworm infection in Thailand.
There is an approximate of thousands infected pet and stray dogs being left unknown annually. It is conceivable that such infected dogs may not be diagnosed thus they continue as a source of microfilariae and transmitted to the mosquito vectors. To be free of circulating microfilariae and to be negative to an appropriated antigen-captured blood test, dogs should be treated on or soon after the positive blood sample has been confirmed. The problem will still leave as small pit where infected dogs may not be successfully detected or be treated.
Key factors which may affect D. immitis infection in Thailand
D. immitis infection appears to widely spread in Thailand and its range is continuing to expand everywhere, from the cities to the villages. There are several key factors which affect the possibility of D. immitis occurrence in Thailand.
Dog population density. To date, the maximum dog population in Thailand is in Bangkok, the capital city. It is proximately 150,000 stray and over 300,000 pet dogs. D. immitis is more likely to be transmitted in the city (urban area) where the density of stray and pet dogs is high. Also it is currently high prevalence of microfilaremic heartworm infection among dog population, 46.17-46.67% in stray dogs and 10.20-11.78% in pet dogs. In the rural areas, dogs represent a smaller proportion of the available hosts on which mosquitoes can feed and the risk of transmission is expected to be lower.
Life expectancy of adults, microfilariae, density of microfilariae and their positive rate in dogs
The long life expectancy of the adult worms approximately 5-7 years and the microfilariae may be as long as 3-6 years. The density of microfilariae in the peripheral blood has been described as nocturnal subperiodic with an inconstant and fluctuated through a 24-hour period (Nithiuthai and Chungpivat, 1991). The highest peak of microfilarial density was at 3.45-5.43 o'clock. Only 5 to 20% of microfilaria was found circulating in the peripheral blood for the rest of the time.
Patent microfilaremia has been observed for many years following the infected stray dogs brought into our animal house. Any infected dogs either stray or pet, can be potential sources of microfilariae for many years. Even if pet dogs treated with an adulticide, it may still pose a risk for a considerable time.
Clinical signs of heartworm infection. Clinical signs may not be apparent for two or more years although microfilariae may be in the peripheral blood circulation. Some dogs may never develop clinical signs if their infections are small. Thus infected microfilaremic dogs may never be diagnosed as such. As clinical signs become more severe, the dog showed progressive signs of a very poor exercise tolerance, dyspnoea, anorexia, ascites and death finally. The most severe condition of heartworm infection, the adulticide treated dogs have usually been seen. Dead adult worms and their fragments became emboli in small pulmonary arteries causing lung damages and other complication. (Jiempittayanuvat et al.,1987). Occasionally, particularly young exogenous breed of dogs which was heavily infected with immature worm showed a caval syndrome and affected dogs died for the unknown reasons. A few cases, immature and adult worms were aberrant parasites in abdominal cavity and blood vessels of limbs. One severe case from a private veterinary practice, an acute inflammation, edematous swelling following by tissue infarction occurred on a front right limb extremity. A number of worms from the vessel were expelled during leg amputation. The adult males and gravid females of D. immitis were identified. Such aberrant migration of heartworm in the blood vessel was abruptly obstructed the blood flow. While other cases the worms were incidentally observed outside the heart during surgical operation without any clinical signs. As indicated above, heartworm disease is a potentially debilitating disease which may severely interfere with the quality of life of infected dogs and interfere with the physical capacity of working dogs and others.
Occult infections. Absence of microfilariae in the blood circulation may be due to prepatent infection, single-sex infection, drug-induced or immune-mediated factors. The occurrence of occult infections has been varied from 10-70% in stray dogs and 30.9% in pet dogs. Occult infections will affect the sensitivity of the diagnosis which relies on detecting microfilariae. Unless the reason is that the low dose of infection of single-sex, drug-induced or immune-mediated, these dogs probably present only a minor risk of disease transmission. However dogs with heartworm infection will carry a certain amount of risk depending on their physical health.
Breed, sex, age and health management of dogs. All breeds of dogs are at risk of heartworm infection from the beginning of life to the old ages. All ages of dogs are at risk of heartworm infection. Both large and small sizes are susceptible to the infection. The ratio of D. immitis infected male to female dogs has not yet been a clear-cut information. Dogs with the indoor lifestyle would have less risk of mosquito exposure than dogs spending their time outdoor. Dogs that live outdoors are high risk of exposure with infected mosquitoes in endemic areas.
Number of infective larvae developing in a mosquito. On average, experimentally, less than 20 infective L3 developed in a single female Aedes aegypti, laboratory strain and commonly recovered only up to 5 larvae. Therefore, a single mosquito bite would usually result in only a small infection in an individual dog. The dog seems likely to be infected on several occasions to get a large number of worms from an infected dog in an area with a high mosquito density. Therefore it is likely that the initial infections with D. immitis to a new dog will be small, may not become clinically apparent, but the parasite will be accumulated over several times of exposure to get heavy parasite loads.
Temperature requirement for D. immitis larval development in mosquitoes and the suitable species of the mosquito vectors. Thailand is a known endemic area for heartworm infection probably due to fact that the average temperatures and relative humidity of the country generally are suitable for larval development through the year. The optimum temperature for larval development was considered to be 22-28°C. Larval development of D. immitis in mosquito would appear to be the most important factor for the successful establishment of this filarial parasite.
Diagnosis of Heartworm Infection. Traditional diagnosis has been relied on identification of microfilariae, usually by concentration methods such as stained buffy-coat blood films, filtration, and modified knott's. Any microfilariae found have to be distinguished, usually on morphology and differential staining. The diagnostic tests of choice presently are those of several commercially available diagnostic test kits (SnapR and WitnessR) which detect circulating D. immitis antigen. These kits generally detect adult female antigen. However, the tests are not capable of detecting a single mature male worm as well as immature infection.
Treatment and Control program for heartworm infection. To ensure that any foci of D. immitis infections in Thailand will be controlled. In endemic area dogs need to be treated prophylactically throughout life. Infected dogs diagnosed with heartworm disease can be treated and this would usually be in the best interest of the animal's health. Initially adult worms need to be eliminated using melarsomine dihydrochloride (ImmiticideR, Merial), the only one adulticide which is available in Thailand. The dose recommended is 2.5 mg/kg intramuscularly injection twice, 24 hours apart. Dogs with high density of microfilariae, severe clinical signs and old ages (over 8-10 years old) are not recommended due to the risk of severe lung injury. Some cases, an additional supportive treatment such as aspirin medication is recommended. Adulticide treatment should be followed at least 4 weeks later with microfilaricidal treatment. The most effective microfilaricide is ivermectin (0.05 mg/kg). Success of adulticide treatment can be monitored with antigen-capture diagnostic kits. Detection of a persistent microfilaremiae is suggested the continuation of existent adult worms.
After a successful treatment of adults and microfilariae of D. immitis infections. A follow-up blood test using an antigen-capture test should be made at 5 to 6 months post-treatment. Heartworm infection can be prevented with medication killing any larvae infecting into dogs within the last 30 days. It will prevent the larvae from becoming adults and causing any clinical signs. Since the weather pattern in Thailand is suitable for survival of mosquito vectors and development of infective larvae in the mosquitoes, it is recommended that uninfected and healthy dogs at high risk area should take heartworm preventive medication monthly. Nowadays, heartworm infection can be prevented by monthly medication which is easily managed by dog owners. Many highly effective drugs are available in Thailand including Ivermectin and Selamectin. Control program by using a proper adulticide and microfilaricide together with regular monthly preventive medication, one would expect to reduce risk of canine heartworm infection for the life extension of healthy dogs in the future.
Acknowledgements
I am very grateful to the technical assistance provided by Drs. Viseshakul and Kalpravidh, Mr. Saiwichai, Ms. Donthong, Chomchai and SongPrakon. I would also like to thank Ms. Chungpivat and Dr. Naraprasertkul who allowed me to unlimited access the information of laboratory results of canine blood parasites used in this paper.
References
1. Choochote, W., Suttajit, P., Rongsriyam, Y., Likitvong, K., Tookyang, B., Pakdicharoen, A., Siriprasert, V. and Sukontasan, K. 1992. The prevalence of Dirofilaria immitis in domestic dogs and their natural vectors in Amphur Muang Chiang Mai, Northern Thailand. J. Trop. Med. Parasit. 15 : 11-16.
2. Choochote, W, Sukhavat, K, Keha, P, Somboon, P, Khamboonruang, C, Suwanpanit, P. 1987. The prevalence of Dirofilaria immitis in stray dog and its potential vector in Amphur Muang Chiang Mai, Northern Thailand. Southeast Asian J. Trop. Med. Public Health. 18 : 131-134.
3. Choochote, W, Somboon, P, Kamboonruang, C, Suwanpanit, P. 1986. A survey for natural vectors of Dirofilaria immitis in Chiang Mai Province, Northern Thailand. Southeast Asian J. Trop. Med. Public Heath. 17 : 146-147.
4. Chuanchuen, R., Klomkleaw, W., Nakawej, B., Nithiuthai, S., Platt, R. and Prechatangkit, B. 1993. Efficacy of an ELISA test kit for canine heartworm antigen detection. Thai J. Vet. Med. 23 : 20-29.
5. Haddock, K.C. 1987. Canine heartworm disease : A review and pilot study. Soc. Sci. Med. 24 : 225-246.
6. Jeimpittayanuvat, S., Chivapat, S.,Tesprateep, T. and Nithiuthai, S. 1987. The efficacy of Ivermectin against microfilariae of Dirofilaria immitis in dogs. J. Thai Vet. Pract. 9 : 115.
7. Nithiuthai, S. and Chungpivat, S. 1992. Lymphatic filaria(Brugia pahangi) in dogs. J. Thai Vet. Pract.4: 123-133.
8. Nithiuthai, S. and Chungpivat, S. 1991. Microfilarial periodiciy of Dirofilaria immitis in the peripheral circulation of dogs. J. Thai Vet. Pract. 3 : 241-247.
9. Salakij, C., Salakij, J., Rochanapat, N., Suthunmapinunta, P. and Nunklang, G. 1999. Hematological characteristics of bloodparasite infected dogs. Kasetsart J.(Nat.Sci.) 33 : 589-600.
10. Sangkavoranond, A. 1981. The prevalence of heartworm (Dirofilaria immitis) in stray dogs from Bangkok Metropolitan area. Kasetsart Veterinarians 2 : 185-189.
11. Sangkavoranond, A. and Paiboolratanawong, S.) 2001. Incidence of helminth infections in alimentary canal and of heartworm infections among stray dogs from Bangkok Metropolitan area. J. Thai Vet. Med. Assoc. 52 : 53-60.
12. Surathint, K., Leemingsawat, S., Tumrasvin, W., Deesin, T., Sucharut, S. and Panvuth, N. 1985. Experimental study on the potential vectors of Dirofilaria immitis in Thailand. J. Parasit. Trop. Med. Assoc. 8 : 40-43.
13. Techodom, J., Chavalkul, S., Taboonpong, S., Nithiuthai, S., Thairoongroj, M. and Prechatangkit, B. 1992. Dirofilaria immitis antigen preparation for the ELISA . Clinical case conference 1992, Faculty of Veterinary Science, Chulalongkorn University. Bangkok, Thailand. 23 pages.