Seven New Cases of Intranuclear Coccidiosis in Tortoises: An Emerging Disease?
Abstract
Intranuclear coccidiosis in tortoises is apparently rare. Only one published account exists, documenting the disease in two captive-bred juvenile radiated tortoises (Geochelone radiata) from St. Catherine’s Island, Georgia, USA.1 Of 93 chelonians submitted to Northwest ZooPath from 1994–1998, only two cases (2.0%) were documented, in a wild-caught adult male impressed tortoise (Manouria impressa) (tortoise 1) and a captive-bred 7-mo-old leopard tortoise (Geochelone pardalis) (tortoise 2). At the Wildlife Conservation Society, two additional cases were documented from adult radiated tortoises from St. Catherine’s Island (tortoises 3–4), and in three Travancore tortoises (Indotestudo forstenii) from an illegal shipment originating in Celebese, that was confiscated by the United States Fish and Wildlife Service (tortoises 5–7). Clinical signs and treatments are summarized in Table 1. Gross and microscopic lesions, distribution of intranuclear protozoa, ultrastructural features and concurrent disease processes are summarized in Table 2. A confirmed ante-mortem diagnosis of intranuclear coccidiosis was not available for any of the tortoises, so specific anti-coccidial drugs were not always administered. Although tortoises were treated with a variety of drugs, a treatment affect was not apparent in necropsy tissues. Systemic infection with intranuclear coccidia was fatal or contributed significantly to death in all cases. Tortoise #1 was unique because intracytoplasmic sporulated stages were detected in some hepatic cells that resembled the sporulated stages of Goussia-like oocysts described in Nile crocodiles (Crocodylus niloticus).2 Tortoise #2 was unique because the coccidia were only detected in the epithelium lining the inner ear and Eustachian tube. Morphologic and ultrastructural features of the parasites indicate that in all cases the organism is a coccidian parasite. These features are similar in tortoises 2–7, and suggest the same species caused infection in these cases. The presence of intracytoplasmic sporulated stages of the parasite in tortoise #1 suggest that this may be a related but different parasite, or that the parasite has a different host relationship in the impressed tortoise. Treatment inconsistencies and lack of ante-mortem diagnoses precluded accurate assessment of a treatment response. The source and route of infection, and predisposing factors for infection have not been determined. Concurrent infectious disease seen in some of the turtles, and evidence of wasting and lymphoid depletion is some tortoises suggest that stress, malnutrition and immunosuppression may be predisposing factors in the development of clinical disease.
Table 1. Clinical signs and treatment protocols in tortoises with intranuclear coccidiosis
|
Tortoise
|
1
|
2
|
3
|
4
|
5
|
6
|
7
|
|
Signs
|
Anorexia, wasting
Several months
|
Anorexia, wasting
4 wk
|
Lethargy, ocular/nasal discharge
11 days
|
Lethargy, ocular/nasal discharge
2 days
|
Lethargy, ocular/nasal discharge
3 wk
|
None
|
None
|
|
Treatments
|
Fenbendazole1
Metronidazole2
Albon3
Ceftazidine4
Chloramphicol5
|
Fenbendazole6
Metronidazole7
Enrofloxacin12
|
Ceftazadime13
Chloramphen.14
Itraconazole15
Fenbendazole16
|
Enrofloxacin17
|
Fluids9
Enrofloxacin8
|
None
Fenbendazole10
|
Metronidazole11
Fenbendazole10
|
1. Panacur, Hoechst-Roussel, Somerville, New Jersey, USA; 50 mg p.o., once, 3 mo prior to death.
2. Flagenase, Laboratorios Liomont, Mexico; 50 mg p.o., 3, 2.5, and 1.5 mo prior to death.
3. Albon Liquid, SmithKline Beecham, West Chester, Pennsylvania, USA; 25 mg p.o., once, 2.5 mo prior to death.
4. Fortaz injectable, Glaxo Pharmaceuticals, Research Triangle Park, North Carolina, USA; 14 mg i.m., s.i.d, 3 to 1 wk prior to death.
5. Chloro Drops, Steris Laboratories, Phoenix, Arizona, USA; s.i.d. for 7 days, 2 wk prior to death.
6. Panacur, Hoechst-Roussel, Somerville, New Jersey, USA; 10mg p.o., once, repeated in 2 wk.
7. Flagenase, Laboratorios Liomont, Mexico; 20 mg p.o., repeated in 2 wk.
8. Baytril, Bayer Co. Shawnee Mission, Kansas, USA 5 mg/kg, p.o., s.i.d. for 7 days, 3 wk prior to death.
9. 2.5% dextrose+0.5% NaCl, 10 cc, s.c., s.i.d. for 6 days, 3 wk prior to death.
10. Panacur, Hoechst-Roussel, Somerville, New Jersey, USA; 50 mg p.o., once, prior to onset of clinical illness (for oxyurids).
11. Flagenase, Laboratorios Liomont, Mexico; 200 mg/kg p.o., duration unknown.
12. Baytril, Bayer Co. Shawnee Mission, Kansas, USA 2.5 mg, i.m., s.i.d. for 10 days.
13. Fortaz injectable, Glaxo Pharmaceuticals, Research Triangle Park, North Carolina, USA; 20 mg/kg i.m., q 72 h, for 11 days, until death.
14. Chloro Drops, Steris Laboratories, Phoenix, Arizona, USA; b.i.d. for 11 days, until death.
15. Sporonox, Janssen Pharmaceutica, Titusville, New Jersey, USA; 100 mg q 48 h, until death.
15. Panacur, Hoechst-Roussel, Somerville, New Jersey, USA; 900 mg p.o. Frequency of treatment not known.
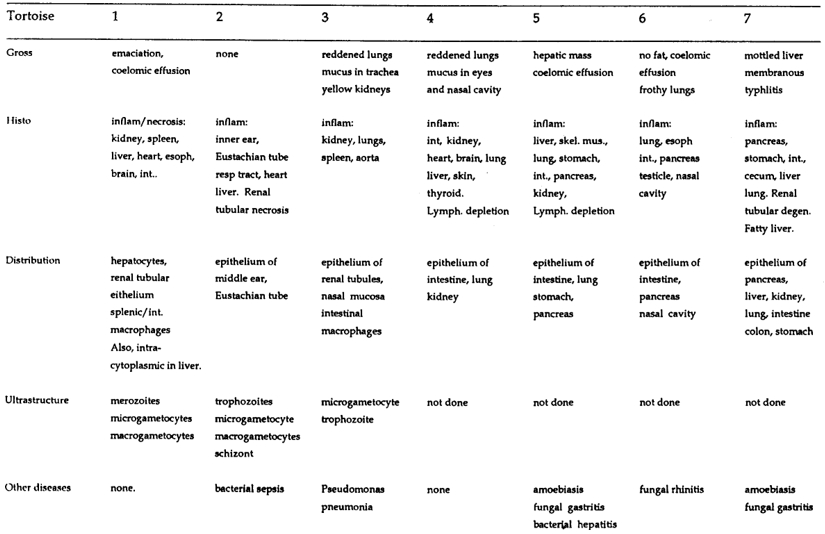
Table 2. Gross and histologic lesions, distribution of protozoa, ultrastructural stages, and concurrent disease in tortoises with intranuclear coccidiosis
Literature Cited
1. Jacobson ER, J Schumacher, SR Telford, Jr., EC Greiner, CD Buergelt, CH Gardiner. 1994. Intranuclear coccidiosis in radiated tortoises (Geochelone radiata). J. Zoo Wildl. Med. 25:95–102.
2. Gardiner CH, GD Imes, Jr., ER Jacobson, CM Foggin. 1986. Sporulated coccidian oocysts resembling Goussia Labbe, 1896 in the viscera of Nile crocodiles. J. Wildl. Dis. 22: 575–577.