Abstract
There are numerous reports of dead captive giraffe with no discernible cause of death except serous atrophy of adipose tissues. The term “peracute mortality syndrome” has been utilized to describe this finding.11 Although the common linolenic acid deficient status of captive animals might contribute to the problem;5 there must be another triggering mechanism. As cold stress is a well-known inducer of lipolysis, the probability of captive giraffe being cold-stressed was examined by a literature search.
In the wild, giraffe are renowned for their ability to withstand extreme heat and radiation.21 In captivity, they rarely seek shade even on hot days.7 Two hypotheses explaining this phenomenon exist: The unusual body shape of the giraffe is believed to maximize heat dissipating surface relative to volume,3 and an obligatory heterothermia allows giraffe to save energy for body temperature (BT) regulation by simply allowing body temperature to rise along with ambient temperature.17,18 The correlation found between ambient and body temperature is demonstrated in Fig. 1. In contrast, the zoo medicine literature gives a constant normal BT of captive giraffe of about 37.5–38.8°C.20
Figure 1. Correlation of ambient temperature and rectal temperature in three giraffe
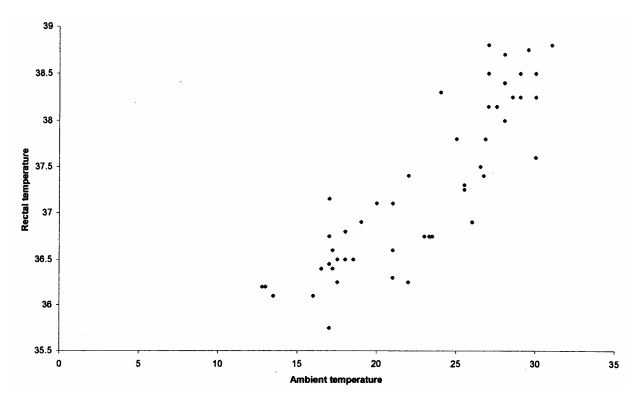
While these adaptations allow giraffe to withstand their share of heat, in theory, the same adaptations make them especially susceptible to cold. If they have a comparatively greater heat-dissipating surface, any circumstance that increases heat convection (e.g., wind or humidity) should have a more pronounced effect on them. If they can depend upon ambient temperature to maintain their BT, and allow it to decrease with nighttime temperatures, any condition that forces them to keep their BT elevated against colder temperatures should impose an additional energetic cost on them-possibly to the extent that cannot be compensated. For free-ranging giraffe, high mortalities have been reported after a period of exceptionally cold and wet weather.29 Gastrointestinal capacity and insufficient diets limit the ability to compensate for the energy demands during these periods.
With prolonged cold exposure, blood glucose (Glc), phosphorus (P) and thyroid hormones (TH) should be increased, while calcium (Ca) and magnesium (Mg) should be decreased.24,26 In a state of prolonged hyperthyroidism, as might be induced by constant cold stress, leukocytosis, lymphopenia, hyperglycemia and hyperphosphatemia can develop.22 Blood values related to the energy status of the animal such as Glc and TH should be related to basal metabolic rate; such a relation has been demonstrated for Glc.28
Selected blood values from free-ranging and captive giraffe are presented in Table 1. In general, captive giraffe sampled appear to have a leukocytosis, lymphopenia, hypocalcemia, hyperglycemia, and hyperphosphatemia. While Glc levels may increase due to handling stress, it is debatable whether these consistent high values can be explained by excitement alone. Mg values are generally unchanged, but the sample size for this mineral was extremely small (n=3). In theory, giraffe showing signs of peracute mortality syndrome might suffer from electrolyte imbalances.
Table 1. Selected blood values in cattle,12 wild9,19 and captive giraffe;4,15,23 ranges for captive giraffe are the range of means from the different sources
|
|
Cattle
|
Wild giraffe
|
Captive giraffe
|
|
WBC × 109
|
4–12
|
-
|
9.8–15.9
|
|
Lymph %
|
45–75
|
-
|
14–34
|
|
Ca (mmol/L)
|
2.1–2.8
|
3.0
|
2.0–2.4
|
|
P (mmol/L)
|
1.4–2.5
|
1.6
|
2.0–3.6
|
|
Mg (mmol/L)
|
0.7–1.2
|
1.1
|
0.9–3.1
|
|
Glu (mmol/L)
|
2.3–4.1
|
3.0
|
4.6–11.3
|
|
Glu (mg/dl)
|
42.1–74.5
|
54.7
|
83.3–203.9
|
A deficiency in linolenic acid, as has been reported for captive giraffe6 could, in theory, contribute to an increase in metabolic rate and diminished adipose tissue stores,13 lymphoid depletion,10 a decrease in both insulin responsiveness and insulin secretion, and a disturbance of myocardial Ca homeostasis.1 A zinc deficiency could, in theory, contribute to a leukocytosis and a lymphopenia,8 and a decrease in adipose tissue stores.14 Both deficiencies should manifest themselves in skin lesions, which have rarely been reported in captive giraffe.
Conclusions
While precise studies are lacking, reported data confirms the hypothesis that captive giraffe should be prone to cold stress. In eland (Tragelaphus oryx), another browsing ruminant with body temperature regulation similar to that of giraffe,27 two cases of death related to stressful events revealed serous atrophy of fat as the only necropsy finding.25 These appear to be the only cases of “peracute mortality” in another ungulate species to date.
Recommendations for indoor housing temperatures for giraffe range from 13–16°C2 to 21–22°C.20 These temperatures are below or close to the ambient temperature that led to minimal BT in free-ranging giraffe.18 A consistent recommendation is that giraffe should not be housed outdoors if temperatures are below 10°C.20 In light of the high incidence of peracute mortality syndrome,16 and its possible link to cold stress, these husbandry guidelines might have to be re-evaluated.
A survey of peracute mortality syndrome related to temperature management and housing latitude is currently being conducted. Fatty acid evaluations are continuing in captive giraffe.
Acknowledgments
The authors would like to thank the staff at the Whipsnade Wild Animal Park, England, and the Kansas City Zoological Gardens giraffe management staff, for conditioning efforts with their giraffe. In addition, a special thanks to Dr. Murray Fowler, whose insight into the initial syndrome, and his contributions to the field of zoological medicine are greatly appreciated.
Literature Cited
1. Ashes, J.R., E. Fleck, and T.W. Scott. 1995. Dietary manipulation of membrane lipids and its implications for their role in the production of second messengers. Proc. Int. Symp. on Ruminant Physiol. 8:373–386.
2. Benbow, G. 1988. Management of giraffe. Proc. Symp. Assoc. British Wild Anim. Keepers and Marwell Zool. Soc. ABWAK, Bristol, England, 74–78.
3. Brownlee, A. 1963. Evolution in giraffe. Nature. 200:1022.
4. Bush, M., R.S. Custer, and J.C. Whitla. 1980. Hematology and serum profiles for giraffes: variations with sex, age and restraint. J. Zoo Anim. Med. 11: 122–129.
5. Clauss, M., E.J. Flach, K. Ghebremeskel, J-M. Hatt, and C. Tack. 1999. Supplementing the diet of captive giraffe with linseed extraction chips. Proc. First Europ. Zoo Nutr. Meeting, 49.
6. Crawford, M.A., P. Budowski, P. Drury, K. Ghebremeskel, L. Harbige, M. Leighfield, A. Phylactos, and G. William. 1991. The nutritional contribution to bovine spongiform encephalopathy. Nutr. & Health 7:61–68.
7. Dagg, A.I. 1970. Preferred environmental temperatures of some captive mammals. Int. Zoo Yb. 10:127–130.
8. Dreosti, I.E., S.H. Toa, and L.S. Hurley. 1968. Plasma zinc and leukocyte changes in weanling and pregnant rats during zinc deficiency. Proc. Soc. Experim. Biol. Med. 128:169-174.
9. Drevemo, S., J.G. Grootenhuis and L. Karstad. 1974. Blood parameters in wild ruminants in Kenya. J. Wildl. Dis. 10:327–334.
10. Fiennes, R.N.T., A.J. Sinclair, and M.A. Crawford. 1973. Essential fatty acid studies in primates. linolenic acid requirements of capuchins. J. Med. Primatol. 2:155–169.
11. Fowler, M.E. 1978. Peracute mortality in captive giraffe. J.A.V.M.A. 173:1088–1093.
12. Fraser, C.M., et al. (eds.) 1991. The Merck Veterinary Manual. 7th ed. Merck & Co. Inc., Rahway, N.J.
13. Holman, R.T. 1968. Essential fatty acid deficiency. Progr. Chem Fats 9:275–348.
14. Huang, Y.S. S.C. Cunnane, D.F. Horrobin, and J. Davingnon. 1982. Most biological effects of zinc deficiency corrected by gamma-linolenic acid but not by linoleic acid. Atherosclerosis 41:193–207.
15. I.S.I.S. Physiological reference values. July 1998. www.worldzoo.org (VIN editor: URL was inaccessible as of 03/10/2021).
16. Junge, R.E. and T.A. Bradely. 1993. Peracute mortality syndrome in giraffe. In: Fowler, M.E. (ed.) Zoo and Wild Animal Medicine. Current Therapy 3. W.B. Saunders Co., Philadelphia, PA, 547–549.
17. Langman, V.A. and G.M.O. Maloiy. 1989. Passive obligatory heterothermy of the giraffe. J. Physiol. 415:89.
18. Langman, V.A., O.S. Bamford, and G.M.O. Mololy. 1982. Respiration and metabolism in the giraffe. Resp. Physiol. 50:141–152.
19. Maloiy, G.M.O., E.T. Clemens, and J.M.Z. Kanau. 1982. Aspects of digestion and in vitro rumen fermentation rate in six species of East African wild ruminants. J. Zool. Lond. 197:345–353.
20. Matern, B. and G. Kloppel. 1995. Giraffe und Okapi. In: Goltenboth, R. and Klos, H.G. (eds.) Krankheiten der Zoo-und Wildtiere. Blackwell Wissenschafts-Verlag, Berlin, Pp. 284-299.
21. Owen-Smith, N. 1988. Megaherbivores. The Influence of Very Large Body Size on Ecology. Cambridge University Press, Oxford, England.
22. Peterson, M.E. 1995. Hyperthyroid diseases. In: Ettinger, S.J. and E.C. Feldman (eds.) Textbook of Veterinary Internal Medicine. 1466–1501.
23. Rhodes, J.A. 1975. Biochemical profiles in exotic captive animals. Proc. Am. Assoc. Zoo Vet. 81–111.
24. Sasaki, Y. and T.E.C. Weekes. 1986. Metabolic responses to cold. In: Milligan, L.P., W.L. Grovum, and A. Dobson (eds.). Control of Digestion and Metabolism in Ruminants. Reston Publ. Co. 326–343.
25. Spengler, R. 1985. Stressbedingte Indigestion bei Elenantilopen. Verh. Ber. Erkr. Zootiere 27:263–264.
26. Sykes, A.R., A.C. Field, and J. Slee. 1969. Cold exposure of southdown and Welsh mountain sheep. 3. changes in plasma calcium, phosphorus, magnesium, sodium, and potassium levels. Anim. Prod. 11:91–99.
27. Taylor, C.R. 1970. Strategies of temperature regulation: effect on evaporation in East African ungulates. Am. J. Physiol. 219:1131–1135.
28. Umminger, B.L. 1975. Body size and whole blood sugar concentrations in mammals. Comp Biochem. Physiol. 52A:455–458.
29. Walker, B.H., R.H. Emslie, N. Owen-Smith, and R.J. Scholes. 1987. To cull or not to cull: lessons from a Southern African drought. J. Appl. Ecol. 24:381–401.