Abstract
A number of wildlife species are known to accumulate excessive amounts of iron stores under conditions of prolonged captivity.4 Recent studies of acquired hemochromatosis in browsing rhinoceroses5 were expanded to determine whether this phenomenon also occurred in another browsing perissodactylid, Tapirus spp.
Fresh and frozen archival sera were obtained from seven Baird’s (Tapirus bairdii), seven Malayan (Tapirus indicus), and four mountain (Tapirus pinchaque) tapirs residing in USA zoos for an average of 13.0 years (range: 0.7–27.0 years). Sera from 13 adult Baird’s tapirs free-ranging in Parque Nacional Corcovado, Costa Rica, obtained over a 30-month period up to February 2000, provided a comparison population of one species subsisting lifelong on natural forage. Serum iron concentrations, total and unsaturated iron-binding capacities, and transferrin saturations were determined by quantitative colorimetry. Serum ferritin concentrations were measured by the enzyme-linked immunosorbent assay developed by Smith et al.6 using reagent standards derived from black rhinoceros ferritin which cross-reacts with both equines and Tapirus spp.
In all three species of captive tapirs, mean serum iron concentrations were approximately twice normal mammalian levels (165, 168, and 229.25 µg/dl for Malayan, Baird’s, and mountain tapirs, respectively). Total iron binding capacities were comparable in all three groups, but transferrin saturations were elevated to means of 60% (Malayan), 64% (Baird’s), and 69% (mountain), again about twice normal mammalian levels. By contrast, mean values for 13 free-ranging Baird’s tapirs were 48.25 µg/dl iron with 33% transferrin saturation.
Serum ferritin assays, which provide the most reliable indirect measure of total-body iron stores, were markedly elevated among all three captive tapir species, but individual values were scattered over a broad range of concentrations. As shown in Fig. 1, there was a general tendency for serum ferritin to increase progressively as a function of time in captivity, and females appeared to have relatively greater concentrations than males. At comparable time intervals, Baird’s tapirs tended to show the highest ferritin values and Malayan tapirs the lowest.
Figure 1
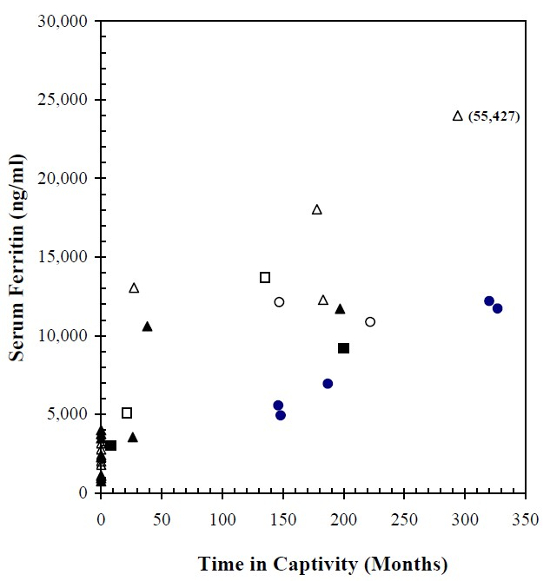
Serum ferritin concentrations in three species of captive tapirs as a function of time in captivity compared to a population of free-ranging Baird’s tapirs (zero time in captivity). Baird’s, mountain, and Malayan tapirs are represented respectively, by triangular, square, and circular symbols (solid for males and open for females).
Captive Baird’s tapirs averaged 17,810 ng/ml (SD 17,130) for all seven animals, or 13,140 ng/ml (SD 2,580) if high and low outliers were excluded. In the captive Malayan and mountain tapirs, mean serum ferritin concentrations were 8,740 ng/ml (SD 3,010) and 7,740 ng/ml (SD 4,100), respectively. By comparison, 13 free-ranging Baird’s tapirs had a mean serum ferritin content of 2,270 ng/ml (SD 1,110). Only one of seven captive Baird’s tapirs had a ferritin value that fell within the range exhibited by free-ranging Baird’s tapirs. Mean ferritin concentrations for the free-ranging tapirs and the entire captive population regardless of species differed significantly (p<0.001).
This pattern of hyperferremia with increased transferrin saturation and marked elevation of serum ferritin in captive tapirs closely resembled that which has been observed among captive populations of black5,6 and Sumatran rhinoceroses.5 Although iron analyte elevations in these tapirs were not as high as seen in many captive rhinoceroses, their magnitudes were sufficient to raise concerns that chronic iron overloads, potentially leading to clinically significant hemochromatosis, may be developing in tapirs maintained under conditions of prolonged captivity. Since increased virulence of invading microorganisms is one of the principal consequences of free iron, its excess might be contributing to the high incidence of infectious diseases affecting captive tapirs.2,3
Progressive iron loading in captive browsing rhinoceroses has been postulated to be caused by increased bioavailability of dietary iron due to deficiencies of natural iron chelators that are normally present in native browse forage, but are reduced or absent in formulated captive diets.5 This postulate may be applied to tapirs as well, since they are also perissodactylids known to consume over 120 species of browse when foraging ad libitum in their natural habitats.1
Quantitative trace metal analyses of necropsy tissues, reviews of histopathology with appropriate stains, and development of species-specific reagents are needed to assess the clinical relevance of these serum analyte measurements. Studies on iron homeostasis in this species may reveal the mechanisms responsible for iron overload, and thereby provide strategies for prevention and/or treatment. Progressive accumulation of excess iron due to unregulated dietary absorption may develop into clinically overt, acquired hemochromatosis, a problem apparently affecting an ever-widening range of wildlife species when brought into captivity.
Acknowledgments
These studies were supported by grants from the International Rhino Foundation (D.E.P.) and the Zoological Society of San Diego (S.H.F.)
Literature Cited
1. Foerster CR, McCoy M. Comportamiento de forrajeo y dieta de una danta Centroamerican en un bosque humedo tropical de Costa Rica. Vida Silvestre Neotopical. 2000 (accepted, under revision).
2. Janssen DL, Rideout BA, Edwards ME. Medical management of captive tapirs (Tapirus spp.). In: Proceedings of the American Association of Zoo Veterinarians. 1996:1–11.
3. Janssen DL, Rideout BA, Edwards ME. In: Fowler ME, Miller RE, eds. Zoo & Wild Animal Medicine. Current Therapy 4. Philadelphia, PA: W.B. Saunders Co.; 1999:562–568.
4. Lowenstine LJ, Munson L. Iron overload in the animal kingdom. In: Fowler ME, Miller RE, eds. Zoo and Wild Animal Medicine. Current Therapy 4. Philadelphia, PA: W.B. Saunders Co.; 1999:260–268.
5. Paglia DE, Dennis P. Role of chronic iron overload in multiple disorders of captive black rhinoceroses (Diceros bicornis). In: Proceedings of the American Association of Zoo Veterinarians. 1999:163–171.
6. Smith JE, Chavey PS, Miller RE. Iron metabolism in captive black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses. J Zoo Wildl Med. 1995;26:525–531.