Abstract
Koi ponds in the United States currently employ space-saving bacterial-based water purification systems. These leave high residual nitrates and dissolved organics in the pond water. Planktonic algal blooms are controlled by UV light units. Steep pond sides and deep water, as well as powerful impeller pumps, have virtually eliminated benthic populations. Water quality problems can only be partially relieved by frequent water changes. A phytoremedial device featuring a live terrestrial plant and artificial benthos in a modular floating unit, was tested in a controlled experiment.
Experimental Design
Two identical tank setups were started with no biologic filtration. One featured the active phytoremedial unit, the other an inert unit.
Results
After 3 months, the test unit showed
1. Absence of ammonia and nitrite spikes (new tank syndrome)
2. Excellent control of nitrates
3. Excellent control of dissolved and particulate organics and planktonic algae as evidenced by turbidity curves and microscopic studies
Conclusion
A phytoremedial device was effective in greatly improving water quality in aqua systems where bacterial filtration was employed and aquatic plants could not be maintained (koi ponds).
Introduction
Traditional koi ponds in Japan and Europe utilize external filter chambers with multiple media, algae and higher plants to achieve water purification. Typically, these filters are large (containing about 0.33 of the volume of the system) and are built adjacent to the pond in full sunlight. Most koi-keepers in the United States favor a naturalistic setting, and the industry has responded with highly efficient bacterial biofilters which are compact (less than 0.1 of the system volume) and designed to be hidden from view, usually devoid of significant plant or algae biomass. Once matured, these filters are very effective at converting ammonia and nitrite to nitrate. They cannot eliminate nitrates and there is little control of dissolved organics. Resulting planktonic algal blooms are controlled by UV light units. Modern koi pond design dictates steep sides and deep water for koi protection and to maximize pond water volume per square footage. Along with powerful impeller pumps, this has virtually eliminated the development of benthic populations. Water quality problems can only be partially relieved by frequent water changes. A phytoremedial device featuring a live terrestrial plant and artificial benthic area in a modular floating unit, was developed for use directly in the koi pond.
Methods
Two identical 10 gallon aquaria were set up, each equipped with a 2 gallon 4” deep refugia mounted adjacent to and slightly above each tank. Tap water was added, buffered with 0.5 tsp CaCO2
(crushed oyster shells) to a pH 7.5 and 100 mg of Na2S2O3 to dechlorinate. A floating phytoremedial device, unplanted and inert, was placed in the control unit refugia. A biologically active unit, consisting of a live Impatiens (Impatiens sp.) grown in a koi pond for several weeks prior to testing, was placed in the test unit. A submersible impeller pump (Supreme Mag-Drive Model 2), fitted with an open cell foam prefilter, was placed in each tank bottom away from the return. Valved to circulate 30 GPH, each refugia was filled via a 1” spillway and returned to the tank via a 1” spillway. This arrangement assured complete maceration of all feces produced, with subsequent delivery to the refugia, as well as highly oxygenated water. Each tank was fitted with a 200 Watt heater (Visi-Therm Aquarium Systems). Tank temperature was maintained at 24°C ± 1. Air temperature varied from a nighttime low of 10°C to a daytime high of 33°C. Water temperature in the refugia varied from 18–26°C. Each tank was stocked with four 6-months-old sibling goldfish (Carassius auratus) size-matched for a total body weight of approximately 230 g. After acclimation, a 33% protein koi food (Mazuri Koi Pond Nuggets) was fed each morning, at a rate of 0.75 g/day. Water was not changed during the test period, but was topped up to replace for evaporation. No artificial lighting was used. Natural filtered sunlight reached both refugia for about 3–4 hours on clear days, otherwise lighting was bright shade. From day 0–52, both tanks were covered with black plastic to limit light entering the water, which was then removed for the remainder of the test period. Water exiting from each refugia was tested for ammonia, nitrite, nitrate, and turbidity (quantitative) each day at approximately 4 hours after feeding. Tests were performed using colorimetric means with either calibrated color discs (Hach FF-2) or photometer (Hach DR/890). Periodically (on days 11, 21, 27, 38, 52, 57, 61, 70, 80, and 84), samples were analyzed microscopically after concentration by low speed centrifugation (approximately 2300 RPM) and staining (Sedi-stain). At the end of the test period, each unit was lifted from the refugia to collect and analyze the evacuated debris microscopically. Light levels were taken periodically on clear days at 1200 hours in direct sunlight (DS), through a south window (SW) and at the level of the experiment (EX).
Results
- Ammonia (Fig. 1) - Photometric verification showed a baseline ammonia production of less than 0.1 mg/day. Subsequent tests were done with color disc analysis.
| Figure 1 | 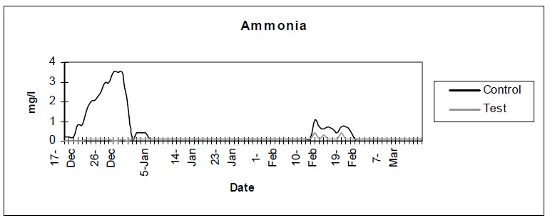
Photometric verification showed a baseline ammonia production of less than 0.1 mg/day. Subsequent tests were done with color disc analysis. |
|
| |
- Nitrite (Fig. 2) - Color disc analysis of nitrite levels.
| Figure 2 | 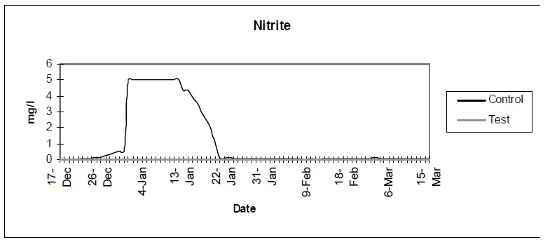
Color disc analysis of nitrite levels. |
|
| |
- Nitrate (Fig. 3) - Photometric analysis of nitrate levels.
| Figure 3 | 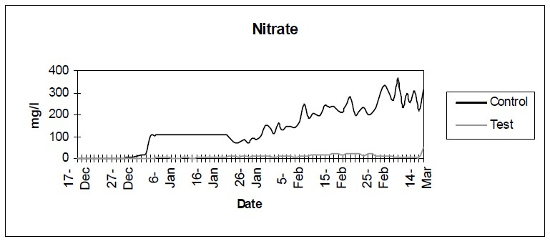
Photometric analysis of nitrate levels. |
|
| |
- Turbidity (Fig. 4) - Photometric analysis of turbidity.
| Figure 4 | 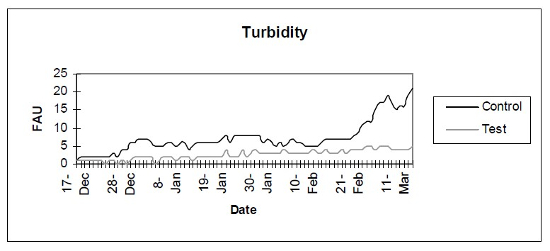
Photometric analysis of turbidity. |
|
| |
- Light levels - day 24 - 19 January - (DS) 90,000 lux; (SW) 70,000 lux; (EX) 6,000 lux day 52 - 6 February - (DS) 130,000 lux; (SW) 120,000 lux; (EX) 10,000 lux
Discussion
The control setup, with an inert device (biologically inactive and unplanted), underwent the typical maturation process known as “new tank syndrome.” Ammonia levels in the control unit rose from day 1, peaked by day 12 and receded to baseline by day 21. The levels in the active unit remained at baseline (Fig. 1). Nitrite levels in the control unit rose from day 1, peaked by day 16, then receded to a low but persistent level by day 36. In the active unit, nitrite appeared at very low levels on day 8 and disappeared on day 12 (Fig. 2). Nitrate levels rose steadily in the control unit throughout the experimental period to peak at 200–300 mg/L. In the active unit, nitrate levels remained very low throughout the test period, peaking at 20–23 mg/L 10–14 days after the plant was harvested. Levels then receded back again to about 4 mg/L by the end of the test period (Fig. 3). These results demonstrated that the active unit could be grown in the same pond as goldfish and marketed along with the fish to deliver immediate biologic filtration. Longer-term nitrate control in the pond is feasible with proper balance of fish, active units, and regular harvesting. Light level readings demonstrate that this plant was able to accomplish this task in very low lighting (6000 lux maximum on clear days). This lighting level was equal in intensity to 4 × 40 watt cool white fluorescents at a distance of 14”, an arrangement very conducive to indoor usage. Fig. 4 shows the quantitative levels of turbidity in both tank-refugia. A surprising finding was that turbidity levels diverged significantly from day 12 on, well before planktonic algal bloom occurred. Microscopically, the main difference was the presence of large and small pieces of detritus in the control water, with almost none in the test water. Subsequent exams (day 21, etc.) revealed the presence of protists (Vorticella, Euglena, Chlamydomonas, etc.) primarily in the control water, with the increasing presence of planktonic algae (mainly Ankistrodesmus, occasional Chlorella) and some filamentous algal chains (Cladophora ?, Mougeotia). The test water showed only occasional planktonic algae. Though always present, the number and size of detrital pieces in the control unit decreased slowly, with minimal effect on the turbidity after day 52. Planktonic algae (Ankistrodesmus) increased in importance, especially after day 52, with readings of 1–3/HPF on day 61, 2–4/HPF on day 70, 5–7/HPF on day 80 and 15–20/HPF on day 84. At the end of the test period, microscopy was performed on the water collected from inside of both units. The water from the control unit was filled with large pieces (0.1–3 mm) of agglomerated organic material. Microscopically, each piece was alive with paramecia, amoeba, nematodes, and oligochaete worms all feasting on the abundant algae. Very strong agglutination was evident throughout the mass. No rotifers could be seen in the water after settling was allowed to occur. The test unit water was relatively free of any macroscopically visible organic matter. However, the water teemed with rotifers (Daphnia) of all sizes.
Conclusions
The unit tested a) successfully prevented spikes of ammonia and nitrite in a newly setup aquasystem (“new tank syndrome”), b) utilized nitrate and allowed for easy export of nitrogen through harvesting, and c) promoted excellent water clarity through the development of an artificial benthic community.