Abstract
Evaluation and safety of new anesthetic techniques depends on critical physiologic monitoring. Blood pH/gas analysis is critical for physiologic evaluation of anesthetized patients. Literature states regimented handling and rapid processing of blood pH/gas samples is required for valid results.1 This is problematic in field situations, especially if numerous samples are collected and analyzed a distance from the anesthetized animal.
This study evaluated three storage conditions on stability of PO2, PCO2, and pH in blood from eight Grevy’s zebras (Equus grevyi). Arterial and venous samples in heparinized 12-ml plastic syringes were stored in ice (ICE) or at 22°C (RT), or with 2 mg/ml sodium fluoride at 22°C (NaF). One analyzer was used for analysis at average times of 20 (baseline), 79, 144, 204, 365, 326, 385, and 452 min.
Data were analyzed using the generalized estimating equation for sequential measure and ANOVA. Mean baseline PaO2 was 81–86 and PvO2 was 49–55 mm Hg. Mean PCO2 and pH in all baseline samples were 61–65 mm Hg and 7.34–7.36 pH units.
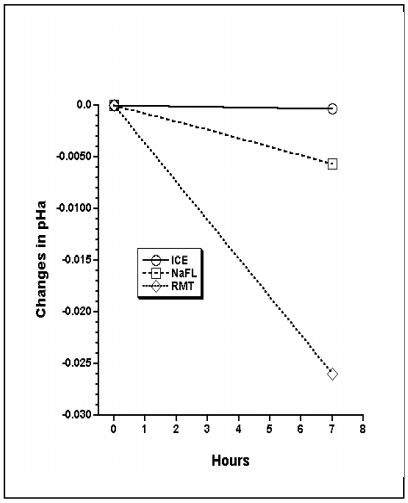
Changes in PaO2, PaCO2, and pHa are shown: Figures 1, 2, and 3.
Zebra blood samples with physiologic blood gases and pH levels are stable in heparin + NaF for up to 7 hr at 22°C.
At RT in heparin alone, PCO2 increases and pH decreases due to metabolism. The increase in PO2 in ICE is from leakage into the syringe. Driving pressure in iced samples is higher than at room temperature because of increased solubility of oxygen, and lower P50 of hemoglobin.2
Figure 1. Change in PaO2 over time
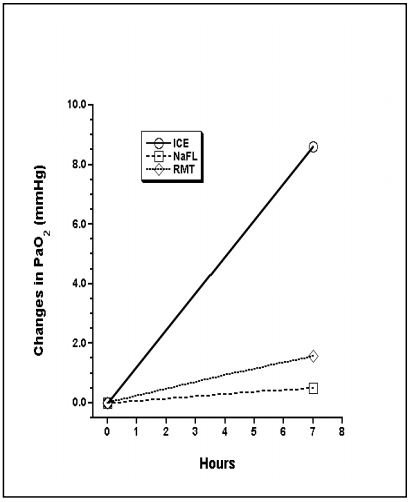
Figure 2. Change in PaCO2 over time
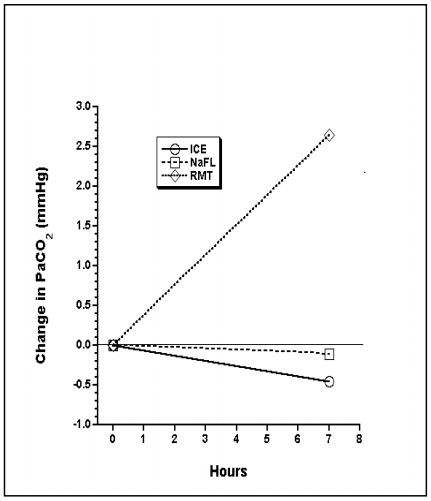
Figure 3. Change in pHa over time
Literature Cited
1. Haskins, S.C. 1977. Sampling and storage of blood for pH and blood gas analysis. J. Am. Vet. Med. Assoc. 170: 429–433.
2. Mahoney, J.J., J.A. Harvey, R.J. Wong, and A.L. Van Kessel. 1991. Changes in oxygen measurements when whole blood is stored in iced plastic or glass syringes. Clin. Chem. 37: 1244–1248.