B. Wright
Gastrointestinal motility disorders are extremely common in the clinic. An excellent clinical practice review was published to discuss this disorder in JVECC (Whitehead, et al. 2016). Most commonly affected are esophageal dysmotility, delayed gastric emptying, small intestinal ileus (functional obstruction), and disorders of colonic motility. These are seen with a variety of critical illness, and many conditions that are primarily gastrointestinal in nature (laparotomy, foreign bodies, pancreatitis, etc.). Symptoms include a lack of auscultated intestinal motility paired with regurgitation or vomiting, abdominal pain, and/or constipation. Increased morbidity and mortality are associated with these conditions, which tend to be incompletely responsive to the available pharmaceuticals. However, judicious use of pharmaceuticals is required, with appropriate awareness of the motility-reducing effects of most antiemetics.
Additional considerations include maintaining appropriate metabolic and fluid balance, early mobilization (exercise/motion), appropriate pain control, and early enteral nutrition. In addition to these recommendations, there is a significant body of work showing the efficacy of acupuncture at various levels of the gastrointestinal tract. These data are primary pre-clinical (rodents) but provide valuable information for the growing use of acupuncture for GI motility disorders in humans (Zhang, et al. 2014).
In addition to specific regional effects on GI motility (esophageal, stomach, small intestine, and colon) changes in visceral sensitivity, GI barrier function, and brain-gut axis have been investigated and reviewed (Li, et al. 2015).
There is significant overlap between the physiologic biochemistry driving gastrointestinal barrier function and the neurochemical mediators that are altered during acupuncture. Acupuncture at ST-36 provided protective effects against gut injury and mucosal barrier dysfunction in hemorrhaged rats by activating the cholinergic anti-inflammatory-dependent pathway and enteric glial cells (Li, et al. 2015).
Finally, acupuncture has solid evidence for reducing nausea (comparable to anti-emetics), and it can thus also serve as an adjunct for promoting early enteral nutrition (Lee, et al. 2015). For all these reasons, acupuncture has a key role in promoting GI function in the ER.
An important additional physical medicine modality to consider for GI dysmotility has already been mentioned: motion/exercise. Exercise is analgesic. Constipation is strongly associated with immobility, and exercise, as well as a routine schedule, is likely to encourage elimination. Additional considerations here include walks in a location that would encourage potty-trained patients to eliminate (such as the outdoors), and perhaps an enclosure to allow leash-free elimination for the shy.
Abstracts from some of the evidence-based research for acupuncture on GI motility, anti-nausea, and gastric mucosal protection are included below:
1) Xinyan Gao, Yongfa Qiao, Baohui Jia. NMDA receptor-dependent synaptic activity in dorsal motor nucleus of vagus mediates the enhancement of gastric motility by stimulating ST36. Evidence-Based Complementary and Alternative Medicine. Volume 2012, Article ID 438460, 11 pages doi:10.1155/2012/438460
- Molecular mechanisms behind efficacy of ST-36 for GI motility disorders.
- 2–3-mA pulse of 0.5-ms duration at a frequency of 4 Hz for 20 min by a pair of needle electrodes inserted 3 mm depth into the skin.
- Control CV-12 (the abdomen point) was also inserted to a depth of 3 mm and stimulated with the same protocol.
- Enhanced NMDAR-mediated synaptic transmission in gastric-projecting neurons of the dorsal motor nucleus of the vagus (DMV).
- Intra-gastric pressure dramatically increased by ST-36 and decreased by CV-12.
- Suppression of presynaptic µ-opioid receptors may contribute.
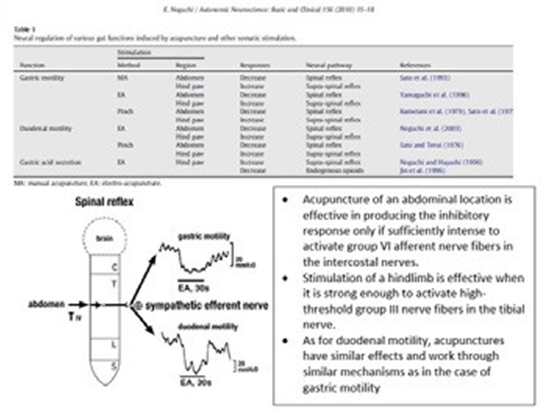
2) Gastric mucosal integrity and healing
Han YJ, Dai WW, Peng L. Effect of acupuncture on contents of beta-endorphin in the plasma and hypothalamus in rats with stress-induced gastric mucosal injury. Zhen Ci Yan Jiu. 2011 Oct;36(5):341–346.
- Four groups: no treatment, injury only, treatment after injury, treatment before.
- Acupuncture was applied to Zusanli (ST-36), Zhong-wan (CV-12) and Neiguan (PC 6) for 20 min, once daily for 5 days.
- Gastric mucosal ulcer index, plasma and hypothalamic beta-endorphin.
- Gastrointestinal propulsion rate was increased remarkably in the prevention group (p<0.05), and the gastric mucosal ulcer indexes and the contents of plasma beta-EP level were decreased obviously in both treatment and prevention groups (p<0.05, p<0.01). The contents of hypothalamic beta-EP were increased.
- Acupuncture of ST-36, CV-12 and PC 6 can promote the repair of gastric mucosal injury and improve gastrointestinal function, which may be related to its effects in reducing plasma beta-EP and upregulating hypothalamic beta-EP level. Acupuncture also has an effect in preventing gastric mucosal injury.
References
1. Lee A, et al. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2015;11:1–137.
2. Li H, et al. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol. 2015;21(27):8304–8313.
3. Whitehead K, et al. Gastrointestinal dysmotility disorders in critically ill dogs and cats. J Vet Emerg Crit Care. 2016;26(2):234–253.
4. Zhang AL, et al. Acupuncture and standard emergency department care for pain and/or nausea and its impact on emergency care delivery: a feasibility study. Acupunct Med. 2014;32(3):250–256.