Senior Lecturer of Veterinary Medicine, The Hebrew University of Jerusalem, Jerusalem, Israel
Heatstroke is caused by the inability to dissipate accumulated heat. In dogs it is characterized by core temperatures above 105.8°F (41°C) with CNS dysfunction. It results from exposure to a hot and humid environment or from strenuous physical exercise. Activation of inflammatory and haemostatic pathways initiates a systemic inflammatory response syndrome (SIRS) which often progresses to multi-organ dysfunction syndrome (MODS). Serious complications of heatstroke include rhabdomyolysis, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC). Several factors are associated with the risk of developing heatstroke. These include prior occurrence of heatstroke or heat stress, obesity, breed (brachiocephalic, Golden and Labrador retrievers), body weight (>15 kg), high environmental temperature and humidity, and lack of acclimation and fitness. Prior heatstroke may affect the thermoregulatory center in the preoptic zone which is responsible for heat sensation and dissipation. Excess body fat increases the body’s natural thermal isolation and impairs normal heat dissipation mechanisms in obese people and animals. Large breed dogs are significantly more at risk of developing heatstroke, particularly exertional heatstroke, suggesting that the ratio between body size and surface area is an important factor of heat dissipation during heat stress. The median body weight of 54 dogs with naturally occurring heatstroke was 31 kg, also supporting this theory. The most common clinical signs of canine heatstroke include collapse, shock, tachypnea, spontaneous bleeding (e.g., petechie, hematemesis, and hematochezia), disorientation/stupor, coma, and seizures. Although the definition of heatstroke is based on hyperthermia causing shock and hypotension, it is important to remember that patients can be hyper-, normo- or hypothermic on presentation, particularly if cooling measures were initiated by the owners prior to presentation. Furthermore, in a retrospective study of canine heat related illness, hypothermia upon admission was a poor prognostic indicator. High body temperatures initiate a myriad of inflammatory, coagulation, and tissue damage processes, varying in severity and progression between dogs. Thermal endothelial cell injury leads to diffuse vascular damage and initiation of coagulation and subsequent microvascular thrombosis. In addition, multiorgan cellular necrosis further stimulates the coagulation system and results in DIC, an important factor in the morbidity and mortality of heatstroke patients. The injured endothelium releases thromboplastin and factor XII, which activates coagulation and the complement cascade, inducing SIRS and widespread DIC. Hepatic injury and failure due to hypoperfusion, microembolism, and direct hyperthermic damage may exacerbate the haemostatic disorders. In vitro studies have shown that high temperatures (>42°C) lead to platelet aggregation, activation of the coagulation cascade, and enhanced fibrinolysis. Normalization of body temperatures inhibit fibrinolysis but not the coagulation cascade or platelet aggregation. In a retrospective study of 54 dogs with naturally-occurring heatstroke, 50% were diagnosed with DIC. In 11 such dogs, severe bleeding and widespread microthrombosis, characteristic of hemorrhagic diathesis were invariably noted at necropsy. As DIC may appear hours to days after the initial hyperthermic insult, dogs with heatstroke should be monitored closely for coagulation abnormalities and clinical signs of DIC for at least 24 hours after the insult. In a recent study in which serial monitoring of coagulation parameters were followed during the first 36 hours of hospitalization in 30 dogs with heatstroke, haemostatic analytes at presentation were not associated with mortality. However, prolonged PT and aPTT at 12–24 hours post presentation, lower total protein C activity at 12 hours and hyperfibrinogenemia at 24 hours post presentation were significantly associated with mortality. Increased D-dimer concentration and low anti-thrombin activity were common at all time-points, but were not associated with mortality (Figure 1) (Bruchim et al. 2016). Interestingly, in that study, which was performed 10 years following the first one at the same institution, DIC was not associated with mortality, the median number of fresh-frozen plasma units administered increased from 2 to 4 units per dog and mortality decreased from 50 to 40%.
| Figure 1 | 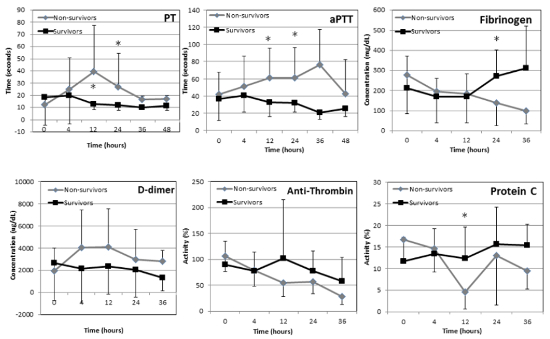
Trends in haemostatic parameters throughout hospitalization in 30 dogs with naturally-occurring heatstroke with survivors (n=18) depicted in black and non-survivors depicted in gray. * depicts significant difference between survivors and non-survivors. PT - prothrombin time, aPTT - activated thromboplastin time. |
|
| |
Heatstroke Complications
Azotemia is a common finding in patients with heatstroke. It results from pre-renal and renal mechanisms, such as severe hypovolemia and direct renal tissue damage leading to tubular necrosis, a frequent finding at necropsy of dogs with heatstroke. Acute kidney injury represents a spectrum of conditions associated with sudden onset of renal parenchymal injury. Heatstroke-associated AKI is likely multifactorial. Such factors include decreased kidney perfusion due to dehydration and hypovolemia, direct thermal injury, myoglobulinemia due to rhadomyolysis, DIC, endotoxemia and the systemic inflammatory syndrome. Kidney injury may be mild and go unnoticed, but often, failure of the kidneys to meet the excretory, metabolic, and endocrine demands of the body ensues. Kidney function parameters can be classified to those measured routinely (e.g., serum creatinine), those not measured routinely, but are calculated using routine chemistry (e.g., glomerular filtration rate [GFR] using endogenous creatinine clearance and fractional electrolyte excretion) and unique urinary renal biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL), C-reactive protein (CRP), and retinol-binding protein (RBP). NGAL is a 25 kD protein, covalently bound to neutrophil gelatinase. Normally, its expression and concentration are low; however, its expression is markedly induced when renal tubular epithelial injury occurs, and it is now identified as one of the earliest and most robustly induced proteins in both human and animal patients. RBP is a low molecular weight protein, freely filtered and is completely reabsorbed by renal epithelial tubules, rendering it a marker of renal tubular function. C-reactive protein (CRP), on the other hand, is a relatively high molecular weight protein, and therefore, is normally not filtered through the glomerulus. Thus, its urine concentration reflects changes in glomerular capillary permselectivity characteristics. In this study we have found that renal biomarker analysis, GFR and sodium fractional excretion can identify kidney damage earlier, immediately at presentation and, therefore, aid the clinicians in the overall assessment of the animal. Nevertheless, the presence of AKI based on the traditional parameters during hospitalization of serum creatinine levels >1.5 mg/dL at 12 and 24 hours after presentation were found to be independent risk factors for death in dogs with heatstroke. Therefore, careful monitoring of renal function and early intervention are warranted.
Severe hyperthermia may lead to cerebral hypoperfusion, neuronal necrosis, direct vascular damage, cerebral edema, hemorrhage, and multifocal vascular thrombosis with tissue infarction that may lead to CNS dysfunction and death. The canine brain is considered more resistant to thermal injury compared to the human brain and other physiological factors, such as respiratory alkalosis, shock, and hypoglycemia, may play a more significant role in the observed CNS clinical signs in canine heatstroke. Thermal and biochemical injury to the pulmonary endothelium may lead to non-cardiogenic pulmonary edema, also known as ARDS. Histopathologic lung lesions in dogs suffering from heatstroke include pulmonary infarcts, marked alveolar hemorrhage or edema.
A few extra-cardiac mechanisms were proposed as contributing processes to the development of cardiac arrhythmias. These included myocardial hypoperfusion, lactic acidosis, and electrolyte imbalance, and possibly direct thermal injury. Post-mortem findings in 11 dogs with heatstroke showed mild to severe subendocardial, myocardial, and epicardial hemorrhages and hyperemia in all dogs.4 These findings suggest that DIC has a pivotal role in the pathogenesis of the reported cardiac arrhythmias. Antiarrhythmic therapy should be considered, however, only if the patient has related clinical signs.
In humans and experimental studies, marked increases in core temperatures are associated with blood flow redistribution, which is characterized by coetaneous vasodilatation that occurs at the expense of decreased intestinal blood flow. This splanchnic vasoconstriction may cause ischemia and limit local vascular heat exchange, thereby promoting bowel tissue hyperthermia. Both intestinal ischemia and hyperthermia may promote oxidative stress that stimulates cytoskeleton relaxation, thus contributing to the opening of tight junctions and/or injuries to the epithelium. These morphological and functional changes enhance intestinal permeability, thus facilitating the translocation of bacteria and endotoxins that are normally contained within the intestinal lumen, and subsequently worsening a systemic inflammatory response syndrome that may culminate in multi-organ system failure and death. Gastrointestinal bacterial translocation has not been specifically documented in dogs with naturally-occurring heatstroke; however, given the massive hemorrhagic diarrhea and hematemesis that rapidly ensues in dogs with severe heatstroke, it is reasonable to assume that it is a major contributing factor to SIRS, sepsis and MODS that may occur in severe cases.
In summary, clinicopathological findings in canine heatstroke are mainly related to the primary thermal insult; however, secondary deterioration occurs due to dehydration, shock, and a poor perfusion to the tissues. Thus, early diagnosis and intervention are crucial to prevent further multi-organ dysfunction and exacerbation of coagulation abnormalities. Time lag from insult to admission (>1.5 hours) was a crucial factor for survival in canines suffering from heatstroke.
Treatment Options
Cooling - whole body cooling prior to admission is highly recommended. The literature provides different cooling methods (e.g., cold enema, gastric lavage, and ice baths); however, other successful and perhaps more practical methods use evaporative cooling via whole body irrigation with tap water and placement of a fan facing the animal. Animals with thick undercoats may benefit from shaving prior to wetting. A cool environment with low humidity is also beneficial. Cooling with ice directly on body surfaces and/or peripheral blood vessels should be avoided as it may result in cutaneous vasoconstriction and decrease heat loss ability. During cooling, the patient’s temperature should be monitored every 5–15 minutes to avoid hypothermia. Cooling should be terminated when body temperature has reached 39.5°C (103°F). Cooling does not result in suppression of the inflammatory response, but will prevent further cellular destruction. Most canine heatstroke victims suffer from distributive shock, as described above. Although the absolute intravascular volume has not changed significantly, vasodilatation and venous pooling of blood lead to a relative hypovolemia. As the animal is cooled, the vasomotor tone will return to normal. Therefore, judicious fluid therapy is warranted. An initial crystalloid dose of 10–20 ml/kg should be administered and perfusion parameters (HR, MM, CRT, pulse quality, blood pressure, mentation, and urine output) continuously reassessed to help guide fluid additional fluid therapy. When perfusion cannot be restored with crystalloids alone, synthetic colloids (hydroxyethyl starch solutions), vasopressor agents (dopamine, vasopressin, and norepinephrine) and positive inotropes (dobutamine) should be considered.
Dextrose should be administered to hypoglycemic dogs as a single bolus (1 ml/kg of diluted 50% dextrose not to exceed a maximum of 10 ml) followed by a 2.5–5% dextrose CRI, with close monitoring of the glucose concentrations. All dogs with heatstroke should be given oxygen therapy during triage. Animals with severe dyspnea or laryngeal edema should be intubated, although this can decrease self-cooling mechanisms inherent with panting. In the most severe cases, general anesthesia with 100% oxygen or positive pressure ventilation may be required. Mannitol therapy may be beneficial in animals with cerebral edema causing intracranial hypertension, although it can also worsen cerebral hemorrhage, if present. Mannitol administration has beneficial effects on the kidney and will help restore urine output and flush tubular casts out in animals with AKI. A suggested treatment regime might include 0.5–1 gm/kg of mannitol over 10–20 minutes after the initial fluid resuscitation, followed by 1–2 additional boluses over the ensuing 12 hours. Benzodiazepines (diazepam, midazolam) are administered as a bolus followed by a CRIif the animal seizures (about 33% of the cases). Other causes for seizures such as hypoglycemia or metabolic and electrolyte imbalances should be ruled-out.
Antimicrobial treatment is not warranted in mild to moderate cases. In severe cases, broad spectrum antibiotics are indicated to treat sepsis due to presumed gastrointestinal bacterial translocation. A combination of antimicrobials effective against gram-positive, gram-negative and anaerobic bacteria is recommended in severe cases utilizing the “escalation-de-escalation” method. A combination of a potentiated penicillin and a fluoroquinolone or a third-generation cephalosporin could be considered. Gastric protectants such as H2 blockers (e.g., famotidine) or proton pump inhibitors (pantoprazole) should be administered to prevent further gastric damage. Antiemetics and promotility agents are essential for prevention of vomiting and consequent aspiration pneumonia. If urine output remains insufficient despite adequate fluid replacement and means arterial blood pressure is >60 mm Hg, medical therapy with furosemide and/or mannitol should be considered. Overhydration must be avoided in anuric/oliguric patients and fluid therapy adjusted based on urine output and intravascular volume status of the patient. Hemodialysis may be indicated in dogs with oligoanuria despite medical therapy, as well as those patients with severe overhydration, uremia, or electrolyte derangements. The treatment of the hemostatic abnormalities due to DIC is based on stabilization of the coagulation system with fresh frozen plasma and concurrent prevention of thrombosis with anticoagulants. Hemofiltration has been suggested as an effective treatment modality in an experimental model of severe canine heatstroke, causing early clearance of accumulated serum cytokines, creatinine, and BUN. Clinical data is unavailable at this time.
Serial monitoring of the patient’s clinical and clinicopathological parameters is essential for early identification of complications and appropriate intervention. Continuous monitoring of vital signs, including temperature, femoral pulse rate, and quality and capillary refill time to assess perfusion, hydration, and shock status, is warranted. In addition, PCV/TS, serum glucose, coagulation profile (including TEG or ROTEM when available), CBC, lactate, blood gas (arterial or venous), arterial blood pressure, and urine output should be monitored. The mental status of the patient should be evaluated frequently and continuous ECG monitoring is recommended as arrhythmias may develop during the first 24 hours after the heatstroke occurs.
Mortality rates in dogs suffering from severe heatstroke are reportedly between 40–50%. Animals with heat-induced illness have a reportedly lower mortality rate (35%). At the author’s institution, mortality rates decreased to 40 and 43% in 2 recent studies (Bruchim et al. 2016; Segev et al. 2015) as compared to 50% 10 years earlier (Bruchim et al. 2006); however, larger scale studies are needed to determine if this trend is real.
In conclusion, heatstroke in dogs is a life-threatening condition, resulting in serious secondary complications such as DIC, AKI, and ARDS, and a high mortality rate despite appropriate treatment. Early admission and treatment along with whole body cooling by the owners and caregivers are important for survival. The diagnosis of canine heatstroke should not rely exclusively on hyperthermia or the presence of neurological abnormalities upon admission, but should be based on the combination of the history, clinical signs, and laboratory results. Treatment and monitoring should be intensive and prolonged since complications can have a delayed onset and present serious risk factors for mortality.
References
1. Krau SD. Heat-related illness: a hot topic in critical care. Crit Care Nurs Clin North Am. 2013;25(2):251–262.
2. Bouchama A, Bridey F, Hammami MM, et al. Activation of coagulation and fibrinolysis in heatstroke. Thromb Haemost. 1996;76(6):909–915.
3. Bruchim Y, Klement E, Saragusty J, Finkeilstein E, Kass P, Aroch I. Heat stroke in dogs: A retrospective study of 54 cases (1999–2004) and analysis of risk factors for death. J Vet Intern Med. 2006;20(1):38–46.
4. Bruchim Y, Loeb E, Saragusty J, Aroch I. Pathological findings in dogs with fatal heatstroke. J Comp Pathol. 2009;140(2–3):97–104.
5. Segev G, Aroch I, Savoray M, Kass PH, Bruchim Y. A novel severity scoring system for dogs with heatstroke. J Vet Emerg Crit Care (San Antonio). 2015;25(2):240–247.
6. Oglesbee MJ, Alldinger S, Vasconcelos D, et al. Intrinsic thermal resistance of the canine brain. Neuroscience. 2002;113(1):55–64.
7. Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988.
8. Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart and Circ Physiol. 2001;280(2):H509–21.
9. Shapiro Y, Alkan M, Epstein Y, Newman F, Magazanik A. Increase in rat intestinal permeability to endotoxin during hyperthermia. Eur J Appl Physiol Occup Physiol. 1986;55(4):410–412.
10. Soares AD, Costa KA, Wanner SP, et al. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Brit J Nutr. 2014;112(10):1601–1610.
11. Chen GM, Lan YY, Wang CF, et al. Clearance of serum solutes by hemofiltration in dogs with severe heat stroke. Scand J Trauma Resusc Emerg Med. 2014;22:49.
12. Drobatz KJ, Macintire DK. Heat-induced illness in dogs: 42 cases (1976–1993). J Am Vet Med Assoc. 1996;209(11):1894–1899.