L. Smart
There has been much interest in the use of intravenous lipid emulsion (IVLE) therapy for many different types of small animal toxicoses in the last 10 years. Although there is some evidence for its efficacy in specific toxicoses, its unbridled use in clinical toxicology is still controversial.
What is IVLE?
Intravenous lipid emulsion therapy involves injection of a fairly large dose of lipid emulsion over a short period of time in order to counteract the effects of particular toxins. It is usually provided as a sterile 20% solution of soybean oil, egg phospholipids and glycerine suspended in water. It is traditionally used for providing a fat source in parenteral nutrition formulations; but in recent times, certainly in our hospital, it is more frequently used as poison antidote therapy. Once the bottle is punctured, it should be discarded within 24 hours due to risk of bacterial growth in the solution. It is ideal to consider each bottle to be single-use only. The IV catheter should be checked for patency before starting the infusion, and the catheter should be flushed well after use. You do not need a dedicated IV line, as it is delivered over a short period of time. However, if using as a continuous-rate infusion (CRI) for more than 1 hour, then attention should be paid to the level of the sterility of the catheter that is used. When considering CRIs, bear in mind that there is no evidence for efficacy of this approach. In general, it is recommended to stop administering IVLE if the serum/plasma is grossly lipaemic.
Mechanism of Action
The use of IVLE for toxicoses had its beginnings as an antidote for local anaesthetic overdose. Initial experimental studies showed improved survival after IVLE in dogs and rats that received a bupivacaine overdose.1,2 Since then, infusion of IVLE during resuscitation for bupivacaine has been reported to be successful in multiple human case reports.3 Bupivacaine decreases cardiac adenosine triphosphate (ATP) synthesis. Lipid emulsion is thought to reverse the cardiotoxicity by providing fatty acids to the myocardium and increasing ATP production, thus improving myocardial contractility. It may also increase intracellular myocyte calcium, also assisting positive inotropy. Since this discovery, the use of IVLE for resuscitation of local anaesthetic overdose has become routine in human medicine.
The second proposed mechanism is creation of a drug-scavenging ‘lipid sink’, into which lipophilic drugs or toxins are absorbed. In theory, absorption of the drug into an intravascular lipid compartment draws the molecule away from its site of action, giving the body more time to metabolise and excrete the perpetrator. The toxin needs to be lipophilic enough, however, for it to be attracted to the lipid compartment. It is difficult to determine what is lipophilic enough. The lipophilicity of any chemical is described as its ability to partition into octanol, expressed as the partition coefficient or log P. In general, a log P greater than 1.0 means that a chemical is lipophilic, and above ∼3.0, it is highly lipophilic. However, there is some variability in the reported log P (depending on how it is measured), and the behaviour of the chemical in vivo is affected by its lipophilicity at blood pH, its volume of distribution and other aspects of pharmacokinetics.4 Therefore, it is difficult to predict if IVLE will be efficacious for drug-scavenging any particular toxin based solely on log P. Generally, the greatest success has been reported for toxins whereby the log P is greater than 5.0.
Previous Reports of Utility in Dogs and Cats
There have been many case reports published in dogs and cats reporting the use of IVLE in toxicoses (Table 1). It is difficult to establish the efficacy of IVLE from case reports, as often multiple other drugs are administered at the same time, and it is unknown whether or not the treatment actually altered the time course of the toxicoses for an individual patient. There is also likely a publication bias whereby instances of perceived efficacy are more likely to be published than lack of efficacy.
Table 1. Case reports in dogs and cats6
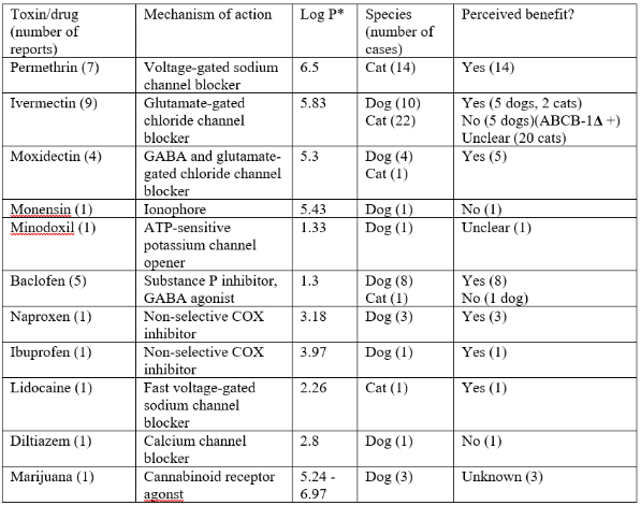
*Retrieved from either https://pubchem.ncbi.nlm.nih.gov or www.drugbank.ca
The types of toxins that IVLE has been used for generally fall into two categories: neurotoxins and cardiotoxins. Most case reports comment on use of IVLE to treat neurological signs due to permethrin or macrocyclic lactone toxicoses. Perceived responses to treatment include various types of neurological improvement, such as reduced tremors, improved mentation or ability to walk, or reduction in sedative drugs needed. There are also some case reports of use of IVLE in toxicoses that have cardiovascular effects, such as lidocaine and diltiazem. However, the information is too limited to comment on perceived benefits.
There is one study worth mentioning in isolation as the only prospective randomised clinical trial on the use of IVLE in toxicoses.5 Thirty-four cats were randomised to receive either IVLE or saline placebo (non-blinded) and then clinically staged for signs of toxicoses over the remainder of hospitalisation. The study found that cats that received IVLE showed faster resolution of clinical signs.
The dose administered in most reports consists of a small bolus (usually 1.5 mL/kg) followed by 8–15 mL/kg over 60 minutes. The use of a bolus was originally designed for resuscitation purposes (i.e., local anaesthetic overdose) and is probably only useful for cardiotoxicity. Use of a bolus for drug-scavenging purposes (i.e., ‘lipid sink’) is questionable and probably unnecessary. The timing of ‘response’ to treatment is usually within several hours of the first dose. Many authors comment on repeating the dose or extending the time of CRI—usually in cases where no initial response was seen.
Adverse Events
The actual rate of adverse events has not been well established, as the evidence relies mostly on case reports. There have been two case reports of corneal lipidosis in cats after IVLE treatment, which resolved within a week, and one report of facial pruritus.5,7,8 Other adverse events reported in human medicine include fat overload syndrome, pancreatitis, cholestasis, acute kidney injury, acute lung injury, venous thromboembolism, hypersensitivity and increased susceptibility to infection.9
Should I Use IVLE?
The benefits versus risk need to be weighed carefully when deciding on whether or not to administer IVLE. In cases where the patient is severely affected by a neurotoxin or cardiotoxin and there is theoretical benefit based on IVLE’s supposed mechanisms of action, then the possible benefit of a single dose of IVLE likely outweighs the risk of adverse effects. There should be some estimation as to whether or not the toxin is likely to still be in circulation, rather than bound to tissue. A repeated dose may be used if there is no response or there is clinical regression; however, the benefit of prolonged CRIs is unknown. In hospitals that regularly use regional anaesthesia (especially bupivacaine), IVLE should be added to the arsenal of emergency drugs available in case of overdose or adverse reaction.
References
1. Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88(4):1071–1075.
2. Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Regional Anesthesia and Pain Medicine. 2003;28(3):198–202.
3. Rothschild L, Bern S, Oswald S, Weinberg G. Intravenous lipid emulsion in clinical toxicology. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2010;18:51.
4. French D, Smollin C, Ruan W, Wong A, Drasner K, Wu AH. Partition constant and volume of distribution as predictors of clinical efficacy of lipid rescue for toxicological emergencies. Clinical Toxicology. 2011;49(9):801–809.
5. Peacock RE, Hosgood G, Swindells KL, Smart L. A randomized, controlled clinical trial of intravenous lipid emulsion as an adjunctive treatment for permethrin toxicosis in cats. Journal of Veterinary Emergency and Critical Care. 2015;25(5):597–605.
6. Robben JH, Dijkman MA. Lipid therapy for intoxications. The Veterinary Clinics of North America: Small Animal Practice. 2017;47(2):435–450.
7. Yuh EL, Keir I. Hypertriglyceridemia and transient corneal lipidosis in a cat following intravenous lipid therapy for permethrin toxicosis. The Canadian Veterinary Journal. 2018;59(2):155–158.
8. Seitz MA, Burkitt-Creedon JM. Persistent gross lipemia and suspected corneal lipidosis following intravenous lipid therapy in a cat with permethrin toxicosis. Journal of Veterinary Emergency and Critical Care. 2016;26(6):804–808.
9. Hayes BD, Gosselin S, Calello DP, Nacca N, Rollins CJ, Abourbih D, Morris M, Nesbitt-Miller A, Morais JA, Lavergne V. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clinical Toxicology. 2016;54(5):365–404.