Update on Allergic Disease: Atopy vs. Contact Allergy
M. Burrows
Animal Dermatology Clinic, Division of Veterinary and Biomedical Science, Murdoch University, Murdoch, WA, Australia
Canine Atopic Dermatitis (CAD)
A definite diagnosis of atopic dermatitis is based on:
- A typical history
- Typical clinical signs
- Rule-out of other pruritic dermatoses
- Allergy investigations
Breed
There are strong breed and/or familial predispositions for CAD, but predisposed breeds vary with geographical regions. Some breeds, such as West Highland White terriers, boxers and bulldogs seem predisposed in all regions; others such as German Shepherds, golden retrievers and Labradors seem to be predisposed for CAD only in some geographic regions. Breed-associated phenotypic variability of CAD has been reported.
Age of Onset
The typical age of onset is reported to be between 6 months and 3 years of age, but occasional dogs develop clinical signs after this age range. Food allergies can commence at any age.
Clinical
The most prominent clinical sign in canine atopic dermatitis is pruritus. In the majority of cases, the pruritus has a characteristic distribution. The pruritus may be seasonal (with CAD) or more typically continuous. Approximately 80% of dogs with seasonal signs are symptomatic in spring or summer, while others exhibit signs in autumn or winter.
Distribution
Generalised or limited to the face, ears, feet, and pinna, and axillae, inguinal region.
Unilateral or bilateral otitis externa.
Pruritus must be present. Early in the disease, some CAD dogs exhibit pruritus with no evidence of any skin lesions. Other cases may have erythema of the affected areas but no other lesions. In some dogs, the initial presentation of CAD is an episode of otitis externa.
In the first stages of the disease, pruritus generally responds well to corticosteroid therapy (0.3 mg to 0.5 mg/kg prednisolone daily).
CAD is a progressive disease. Most of the clinical signs develop due to self-trauma and/or secondary infections. Chronic changes of CAD include alopecia, excoriations, hyperpigmentation, lichenification and/or signs of secondary bacterial (papules, pustules, crusts, erosions, epidermal collarettes) and/or yeast (hyperpigmentation, lichenification) infections. Recurrent ear infections are frequently observed. It is important to recognise these because they may require specific management in addition to control of the allergic dermatitis.
Other historical features help to support the diagnosis of CAD, such as sneezing, reverse sneezing and conjunctivitis. A lack of gastrointestinal signs in a dog with pruritus compatible with ACD also helps by decreasing but not eliminating food as a cause.
Diagnosis
The diagnosis of canine atopic dermatitis is based on typical history and clinical presentation and most importantly by exclusion of other pruritic skin diseases.
Pruritus, the defining feature of canine atopic dermatitis, is a common presenting problem for veterinarians. Pruritus is a sensation in the skin that occurs with a number of skin diseases; however, the list of differential diagnoses for common causes of pruritus that appear clinically similar to CAD is relatively small. Causes of pruritus are often classified into primary diseases, able to cause pruritus directly, or secondary causes which are diseases (usually infections) that occur as a consequence of the damage to the skin caused by a primary disease. The possibility of several co-existing primary and/or secondary pruritic diseases should be considered. In particular, for the diagnosis of CAD, it is important to determine if the pruritus and erythema are the results of environmental CAD, and/or the presence of hypersensitivity to fleas, and/or adverse reaction to food, and/or infections. Often a variety of therapeutic or diagnostic trials are indicated to really determine the cause of the clinical signs of dogs with pruritus. Allergy testing is not considered to be a major tool in the diagnosis.
Key Points for the Diagnosis of Atopic Dermatitis
1. Assess the distribution of the disease
a. Paws, especially ventral interdigital
b. Concave base of pinna
c. External orifice: ears
d. Flexor surface metacarpal or metatarsal
e. Flexure of the elbow
f. Axilla
g. Abdomen/inguinal
h. Periocular
i. Perioral
2. Look for skin lesions
a. Erythema
b. Check carefully for papules (secondary infection, flea bites, scabies or contact dermatitis)
c. Check carefully for bacterial infection (papules, pustules, crusts, erosions, epidermal collarettes)
d. Check carefully for yeast infection (erythema, scale, hyperpigmentation, lichenification, greasy exudate, odour)
3. Look for fleas
a. Acute: macules, papules, crusted papules, hot spots
b. Chronic: alopecia, lichenification, hyperpigmentation
c. Flea therapeutic trial: oral daily nitenpyram
4. Evaluate for food allergy
a. Age of onset <12 months
b. Non-seasonal (cf seasonal for CAD/FBH)
c. Gastrointestinal signs
d. Elimination diet trial
If the dog is still pruritic after the above conditions have been ruled out or controlled, and it has compatible clinical signs, then it is usually possible to make a tentative diagnosis of atopic dermatitis.
At this point the clinician has two options: either symptomatic treatment or allergy testing. Which route is taken depends on a number of factors including the severity of the pruritus, age of the dog, financial considerations, and what the owner wishes for their pet. In some cases, symptomatic treatment may be recommended at the beginning and then allergy testing performed at some point in the future.
Allergy Testing
Two methods of allergy testing are routinely available for the further investigation of canine atopic dermatitis: intradermal allergy testing (IDAT) and serum in vitro testing (SIVT). The diagnostic accuracy of either test is low because there are multiple allergens that can give positive results in clinically normal dogs and dogs with other skin diseases. The test result is only meaningful if the dog has clinical signs consistent with atopic dermatitis and all other pruritic diseases have been ruled out. They are useful tests if owners wish to consider immunotherapy as a management strategy for the dog with chronic allergic dermatitis.
In summary, allergy testing is recommended after a clinical diagnosis of CAD is confirmed in patients where ASIT is indicated and reduction in pharmacotherapy is desirable in a dog with a compliant temperament and a motivated client.
Intradermal Allergy Testing (IDAT)
A variety of companies manufacture allergens for IDAT; however, most dermatologists currently utilise allergens from Greer Laboratories (Lenoir, NC, USA) and dilute according to the manufacturer’s recommendations with a final allergen concentration of between 1000 and 1800 PNU/ml (protein nitrogen units/ml) for dogs. Most veterinary dermatologists test for reactivity against the following antigens: house dust mite and storage mite antigens (Dermatophagoides farinae, Dermatophagoides pteronyssinus, Acarus siro, Tyrophagus putrescentiae); insect body parts/faecal elements (cockroach, moth, ant, houseflies); pollens (from trees, weeds and grasses); moulds (from the household or from crops) and Malassezia.
The inclusion of regional allergens (pollens) in the testing kit is based on knowledge of the plants in a particular geographical location. The intradermal allergy test is interpreted by correlating the positive reactions with the patient’s history. Clinically relevant reactions can then be used to choose allergens for specific immunotherapy.
Intradermal testing is best performed with the dog under sedation in lateral recumbency. Medetomidine (Domitor®) at a dose of 5 to 10 mcg/kg IV is the preferred sedative. Acepromazine is not acceptable because it reduces skin test reactivity. A patch of fur is clipped from the lateral thorax (15x10 cm), and the injection sites are marked with a black marker pen.
A standard intradermal injection of 0.05 ml per allergen is injected intradermally along with the positive (histamine) and negative (saline) control. The reactions are read 10 to 20 minutes later. These appear as wheals. The reactions are subjectively graded based on wheal diameter, height, turgidity and erythema and graded from 0 to 4.
A score of 0 represents a negative reaction (equivalent to the negative saline control), and a score of 4 represents a positive reaction that is equivalent to the positive histamine control. In some cases, late-phase reactions may occur at some sites 24 to 48 hours later. These appear as erythematous, indurated areas that may contain a papular eruption. The full significance of these reactions is currently unknown.
A positive reaction requires functional cutaneous mast cells and the presence of allergen-specific, mast-cell bound, reaginic (presumed IgE) antibodies. Allergenic epitopes cause dimeric or trimeric cross-linking of IgE with resultant release, pharmacologically reactive substances such as histamine, serotonin, and various leukotrienes. An immediate reaction is mediated by histamine and neurogenic factors while histamine, prostaglandins and other vasoactive amines are involved in late-phase reactions.
| Intradermal allergy testing | 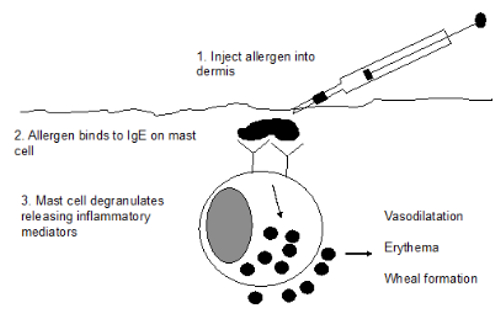
Reproduced with permission from Dr. Peter Hill and Zoetis: Australian Veterinary Dermatology Advisory Panel Guidelines for the Diagnosis and Management of Pruritic Dogs |
|
| |
Allergen solutions are expensive, and it would not be cost effective to offer this service unless one or two tests per week were being performed. Practitioners interested in performing this procedure should study for a further qualification in the discipline or undertake residency training.
Serum in vitro IgE Allergy Testing (SIVT)
In vitro testing simply requires a blood sample to be collected and sent off to an appropriate laboratory. The serum is assayed for allergen-specific IgE, and the results are reported as relative units (the higher the score, the higher the level of IgE). The in vitro test is interpreted by correlating the positive reactions with the patient’s history. Clinically relevant reactions can then be used to choose allergens for specific immunotherapy.
The diluted serum sample is added to a plate containing individual wells coated with specific antigens. The types of antigens tested are similar to those used for intradermal allergy testing, but there are usually less in a skin test. If there is any IgE in the serum that is specific for a particular antigen, it binds to it. The bound IgE is then detected by adding an enzyme-linked reagent that can bind to IgE. This is either a monoclonal antibody or a receptor for IgE molecules. A substrate is added that changes colour when it contacts the enzyme attached to the IgE reagent. The degree of colour change is proportional to the amount of IgE that is bound. The colour change is measured by an automated reader, and the results are reported as a numerical score. The significance of various scores is indicated by the laboratory.
| In vitro measurement of allergen-specific IgE | 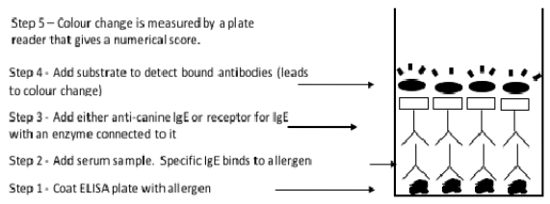
Reproduced with permission from Dr. Peter Hill and Zoetis: Australian Veterinary Dermatology Advisory Panel Guidelines for the Diagnosis and Management of Pruritic Dogs |
|
| |
The ELISA (enzyme-linked immunosorbent assay) most commonly used in Australia is the Heska ALLERCEPT™ assay. The ALLERCEPT™ ELISA assay uses the alpha chain of the high-affinity FcεRI receptor as its detection reagent to ensure specificity for IgE.
Preparation of Animals for Allergy Testing
Before either of the above tests are performed, it is important that the patient is adequately prepared. Clinicians should ensure that:
- Other pruritic diseases have been ruled out
- Anti-pruritic drugs have been withdrawn for a suitable period of time (Table 1)
Table 1
|
Treatment
|
Intradermal testing
|
In vitro testing
|
|
Methylprednisolone acetate injection
|
6–10
|
3–5
|
|
Daily prednisolone
|
4–6
|
0
|
|
Alternate-day prednisolone
|
4–6
|
0
|
|
Topical steroids (including ear and eye drops)
|
1
|
0
|
|
Antihistamines
|
1
|
0
|
|
Cyclosporin
|
0
|
0
|
|
Essential fatty acids
|
0
|
0
|
|
Oclacitinib
|
0
|
0
|
Note: Treatment with methylprednisolone, daily and every other day prednisolone, or cyclosporin for periods longer than 3 months may require longer withdrawal times.
Which Test is Better for Selecting Allergens for ASIT?
Veterinary dermatologists are often asked which is the better test. When answering this question, it is important to remember that the tests are not measuring the same thing. In vitro tests merely measure the amount of allergen-specific IgE that is present in the blood. Intradermal allergy testing detects the presence of allergen-specific IgE that is bound to mast cells in the skin. However, intradermal allergy tests also measure mast cell releasability (this can be altered in atopic dermatitis) and the response of the skin to inflammatory mediators. Intradermal allergy tests, therefore, provide a complete functional assessment of some of the pathways that are required to initiate an allergic reaction in the skin. In contrast, in vitro tests only measure one particular point in the pathway. For this reason, most veterinary dermatologists regard intradermal allergy testing as the superior test.
If it is not possible for a dog to undergo intradermal allergy testing (e.g., if the practice doesn’t perform it, there is no local referral centre, the owner doesn’t want referral), in vitro tests can be used as alternatives to identify allergens for use in immunotherapy.
Despite the above theoretical and practical considerations, it is common for a positive reaction to occur in one test and not the other. Performance of both tests at the same time is more informative, although it may be cost prohibitive. Of note, an increase in the efficacy of the chosen immunotherapy based on the combined test results has not been confirmed in properly controlled studies.
Contact Allergy
Allergic contact dermatitis is commonly suspected in dogs, but establishment of a precise diagnosis can be challenging. The most common plant contact allergens are grasses (Cynodon and Kikuyu species), plants (Tradescantia spp.) and other members of the Commelinaceae (succulent ground covers) family. Other causes of contact allergy include topical antibiotics (neomycin), vehicles used for topical preparations (propylene glycol), shampoos (chlorhexidine), flea products, carpet deodorizers and metals.
Clinicians should be aware that there is considerable overlap between the clinical appearance of atopic dermatitis, food allergy, staphylococcal pyoderma, Malassezia dermatitis and contact dermatitis. It can be difficult to diagnose and may be frequently misdiagnosed as atopic dermatitis. In many cases, contact allergy actually co-exists with atopic dermatitis, and this makes the diagnosis complex and difficult.
Age of Onset
The typical age of onset is reported to be adult dogs of 6 months and older, although it has been diagnosed in dogs as young as 2 months of age.
Clinical
Distribution
Contact allergies typically affect the sparsely haired regions of the face (muzzle and periocular region), concave pinnae, inguinal area, feet, perineal and genital area (plants or carpets) and scrotum (floor detergents, cement, bleach).
Lesions
Lesions are particularly evident on glabrous areas. Intense pruritus is common and in severe cases can lead to a lack of response to anti-inflammatory dosages of corticosteroids. A primary erythemic maculopapular eruption is visible in affected areas. Self-trauma and chronic inflammation may lead to hyperpigmentation and lichenification.
Diagnosis
The diagnosis of contact allergy is usually based on the combination of clinical signs and response to confinement followed up by scratch or patch testing. Interpretation may be complicated by the fact that animals may have more than one condition at the same time, some of which wax and wane. An integrated and sequential investigation is usually required if successful results are to be achieved.
Diagnostic tests can be used to investigate suspected contact dermatitis:
1. Removal of suspected causes of contact irritation: Collar, plastic food bowl, topical medication, shampoo or floor disinfectant.
2. Environmental restriction: Bathe and isolate dog in a new environment (kennel or hospitalisation) for allergen avoidance. Complete resolution of the lesions within 7 to 10 days following restriction suggests that contact dermatitis may be involved; re-challenge to confirm the diagnosis and lesions should recur within 1 to 4 days.
3. Scratch or patch testing: Permits identification of the allergen (kikuyu grass, buffalo grass, Tradescantia spp. etc.) and is usually performed after confinement.
Scratch or Patch Test
A scratch/patch test can then be performed to identify specifically what the trigger is. A patch of skin is clipped over the lateral thorax. The test sites are then outlined with a marker pen. The suspected surfaces are then rubbed onto the skin. A scratch to the skin surface can be made using a 23-G needle to ensure penetration of the stratum corneum.
Topical medications and shampoos can be applied in their normal formulations, powders can be mixed with petroleum jelly, and floor cleaners and disinfectants can be applied at their normal working dilutions. Plant extracts can be made using a mortar and pestle. The sites are then monitored for signs of erythema, oedema and pruritus. The test should be read at 15 to 20 minutes looking for immediate reaction while the substance is still on the skin. The test solution is not washed off. Owners should be asked only to gently wash or wipe the dried solution off with tap water after 24 hours and then to observe the test site for redness or rash over the next 24 to 48 hours.
Treatment
Immunotherapy is not effective. The best approach is avoidance. When avoidance is not feasible, glucocorticoids can be used either topically or systemically to minimise the severity of clinical signs. Pentoxifylline has been reported to be effective for contact allergies but works best as a preventative rather than treatment and should be started 48 hours prior to exposure.
References
1. Griffin C. Diagnosis of canine atopic dermatitis. In: Noli, Foster, Rosenkrantz, eds. Veterinary Allergy. John Wiley and Sons; 2014:70–77.
2. Favrot C, Steffan J, Seewald W, et al. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatology. 2010;21:23–31.
3. Marsella R. Contact allergy. In: Noli, Foster, Rosenkrantz, eds. Veterinary Allergy. John Wiley and Sons; 2014:185–190.
4. Picco F, Zini E, Nett C, et al. A prospective study on canine atopic dermatitis and food induced allergic dermatitis in Switzerland. Vet Dermatology. 2008;19:150–155.