Senior Lecturer of Veterinary Medicine, The Hebrew University of Jerusalem, Jerusalem, Israel
Pathophysiology
Coagulation disturbances are common in emergency and ICU patients. It may be a sequel of various etiologies including intoxications (rodenticide intoxication), snake bite, GDV, pancreatitis, severe trauma, sepsis (e.g., septic peritonitis, pyometra, pyothorax) or any etiology associated solely with thrombocytopenia (IMT, ehrlichiosis). Although all the aforementioned etiologies carry various coagulation disturbances severity, clinical presentation, treatment, prognosis, and outcome, they all share in common inflammatory process so called systemic inflammatory response syndrome (SIRS). SIRS and sepsis are thought to be intimately associated with coagulation system (Figure 1). The mortality is due to organ failure and increases with the number of organ system involvement and failure. Much of the organ failure is due to the microvascular and coagulation disturbances leading to enhance thrombosis and disseminated intravascular coagulation (DIC). The coagulation disturbances are dynamic and variable, depending on the etiology and the severity of the primary disease. Cytokine production, vascular damage, and the release of tissue factor all contribute to the stimulation of coagulation cascade, down regulation of the fibrinolytic system, and consumption of the endogenous anticoagulant.
| Figure 1 | 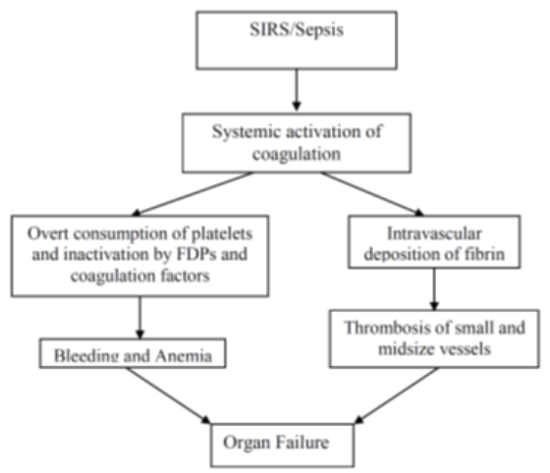
The mechanisms leading to the development of disseminated intravascular coagulation. |
|
| |
In healthy animals, the normal clot formation and fibrinolysis mechanisms are well balanced, so coagulation and formation of clots occur only on demand. However, during massive trauma, infective, inflammatory/septic state this balance may be disrupted, leading to concurrent excessive clot formation and bleeding (Figure 1). Thus, DIC may be considered as an uncontainable burst of thrombin generation and activation, resulting in systemic fibrin formation, followed by plasmin and kinin activation, with simultaneous suppression of the physiologic anticoagulation mechanisms and delayed fibrin removal as a consequence of impaired fibrinolysis (Figure 2).1-3 During this excessive intravascular coagulation phase, platelets, and coagulation factors are consumed, resulting in thrombocytopenia, thrombocytopathy, and depletion and inactivation of coagulation factors.
DIC is categorized into bleeding, organ failure, and non-symptomatic types according to the sum of vectors for hyper coagulation and hyperfibrinolysis. There are 3 types of clinical presentation of DIC; bleeding, massive bleeding, and the organ failure hypofibrinolytic type.4
| Figure 2 | 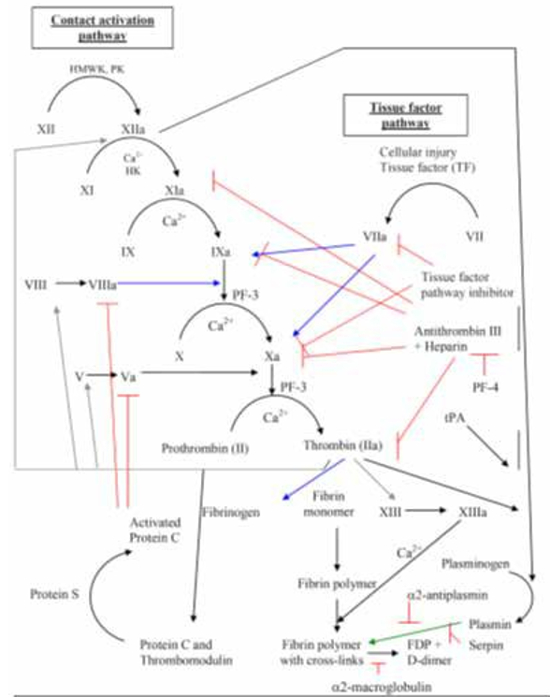
Coagulation pathways. Contact activation pathways and tissue factor pathways formally known as the intrinsic and extrinsic pathway respectively
PK - Prekallikrein; HMWK - high molecular weight kininogen; tPA - tissue plasminogen activator; FDP - fibrin degradation products; Serpin - serine protease inhibitor; PF-3, 4 Platelet factor - 3, 4; The letter “a” following factor number (e.g., Xa) indicates activated factors. Arrows: Black - conversion activation of factors, Red - inhibition action, Blue - reaction catalyzed by activated factor, Gray - actions of thrombin, Green - lyses. |
|
| |
The Bleeding, Massive Bleeding, and Hypofibrinolytic Types
When the dominant feature of haemostatic disorder is hyperfibrinolysis, bleeding is the primary syndrome, therefore, is called the bleeding type. This form of DIC is often seen in patients with major trauma, neoplasia (e.g., hemangiosarcoma, leukemia, lymphoma) and obstetric disease in women. In the other hand when the hypercoagulation is the more remarkable process along with hypofibrinolytic, organ failure is the common symptom. This type of DIC is called the organ failure type due to intravascular clot formation and deposition, resulting in organ failure commonly liver, kidney, and CNS. This type is common in infectious diseases, particularly sepsis.4
The third type is the massive bleeding type or so-called consumptive type, when both coagulation and fibrinolysis are highly activated resulting in whispered clot formation and massive bleeding in the same time, which may result in acute death. This form of DIC is seen in patients with heatstroke, snake bite, and severe acute necrotizing pancreatitis or any major trauma.
When both vectors of coagulation are weak (coagulation and fibrinolysis) there are mild to non-clinical signs directly related to the coagulation disorders, commonly seen in different neoplastic diseases in which only laboratory coagulation parameters are mildly abnormal, and there is a new chronic coagulative balance, that can deteriorate to one of the above described clinical state of DIC.4
Sepsis and Coagulation
The most common etiology involved in coagulation abnormalities in both human and veterinary medicine ICU is sepsis. Sepsis is one of the oldest and most elusive syndromes in medicine. However, with the advent of modern antibiotics, germ theory did not fully explain the pathogenesis of sepsis: many patients with sepsis died despite successful eradication of the inciting pathogen. In addition, it was noticed that other etiologies not infective resemble sepsis in the clinical signs; tachycardia, tachypnea, elevated/decreased white blood cells count, hypoglycemia, with no apparent infectious site (e.g., heatstroke, trauma, cancer). Thus, researchers suggested that it was the host, not the germ that drove the pathogenesis of SIRS/sepsis. The incidence of sepsis increased with the use of immunosuppressive drugs, chemotherapy, and invasive procedures. It is a leading cause of death in humans and in the veterinary medicine with high mortality rate of 30–50%. Common diseases associated with sepsis in the veterinary medicine are pneumonia, pyothorax, peritonitis, pancreatitis, prostatitis, and wound infection.
During inflammation there is increase in plasminogen activator factor inhibitor I (PAI-I) induced markedly increase levels cytokines and liposaccharide, in the blood as a cause of hypofibrinolysis, with consequence of thrombus formation and deposition. Moreover, histones are highly conserved, positively-charged nuclear proteins, serving as the basic structure block unit of the chromatin, leak from damaged and activated immune system cells (e.g., neutrophils and mast cells) and by neutrophil extracellular traps (NETs) into the extracellular space, exhibiting toxic, pro-inflammatory, and pro-thrombotic properties. Histones promote the apoptosis of vascular endothelial cells and platelet aggregation enhancing thrombus formation.5
Clinical Signs
Dogs with DIC may present with several clinical presentations. Three phases of DIC are recognized: the peracute hypercoagulable phase, the acute consumptive phase, and the chronic silent phase. Both the hypercoagulable and the chronic silent phases are non-overt. The latter phase appears to be common in dogs with malignancy and other chronic disorders. The peracute and acute phases may result from an acute phenomenon (e.g., sepsis, acute pancreatitis, heatstroke, electrocution), or it represents acute decompensation of the chronic silent process.6 Acute DIC is extremely rare in cats. Regardless of the pathogenesis, dogs with acute DIC are often presented for treatment due to profuse spontaneous bleeding, either primary (petechiae, ecchymoses, hematochezia, melena, hematemesis, and hematuria) or secondary (blood in body cavities) in concert with hemostatic disorders, and constitutional signs, secondary to anemia or to parenchymal organ thromboses leading to multiple organ dysfunction.2, 6-8 Clinical signs may be highly variable depending on the underlying primary disease and the phase of DIC (i.e., the balance between thrombosis and hemorrhage). Most cats with DIC do not show evidence of spontaneous bleeding and the clinical signs are often those associated with the primary disease.3
Diagnosis
Diagnosis is complex and should be based on several and continuous blood coagulation parameter and clinical signs. The definition and diagnostic criteria of DIC in veterinary medicine are somewhat controversial; however, it is generally agreed that DIC is suspected when an underlying clinical condition known to precipitate DIC occurs, clinical signs of bleedings tendencies (e.g., hematochesia, melena, epistaxis, etc.) with at least one abnormality from the 3 following coagulation groups; 1. Thrombocytopenia <150,000, 2. Coagulation parameters; prolonged prothrombin time (PT), activated partial thromboplastin times; aPTT and activated clotting time (ACT), 3. Inhibitor consumption; decreased protein C, protein S or antithrombin activities; PCA, PSA, and ATA, respectively), and increased fibrinolysis; hypofibrinogenemia, increased D-dimer and fibrinogen degradation products concentrations (FDPs).3 (Table 2)
Table 2. Laboratory screening tests for DIC in dogs and cats
Legend Table 2. Laboratory results should always be interpreted with caution, in light of the history and clinical signs, since an abnormality in any single test is not specific for the diagnosis of DIC. These tests are not very sensitive markers of DIC, and may yield normal results in the early hypercoagulable stages of DIC. Serial monitoring of laboratory tests to assess the trends in individual DIC-suspected patients is useful.
*Depends on the underlying disease.
Thromboelastography (TEG)
This technique characterizes the coagulation function by recording a tracing that represents blood clot creation and breakdown. The tracing is a sum of the interactions between coagulation factors, platelets, fibrin, fibrinolysis, and time. Different thromboelastographic patterns have been identified in a variety of haemostatic disorders, including coagulation factors deficiency, thrombocytopenia, increased fibrolysis, and hypercoagulability.9 In septic human patients TEG has been utilized to identify the hypercoagulable state that precedes the clinically recognizable phase of DIC.10 In veterinary medicine, TEG has been evaluated in a number of studies in dogs and cats.11-13
Treatment
Treatment should be instituted immediately once a diagnosis of DIC has been established or when a high index of suspicion is present. Removing or eliminating the precipitating cause constitutes the cornerstone and the main therapeutic goal for patients with DIC. The wide variety of underlying disorders makes the therapeutic approach to DIC particularly difficult.14
The treatment aims for DIC in dogs and cats include the following:
1. Impeding, possibly stopping, intravascular coagulation and hemorrhage
2. Maintaining good parenchyma organ perfusion
3. Preventing secondary complications
4. Use of anticoagulant - heparin
5. Use of antifibrinolytic agents - tranexamic acid
Replacement therapy is the mainstay for the treatment of DIC.14 A dual approach is used to halt intravascular coagulation:
Blood Component Therapy
Administration of fresh or fresh-frozen plasma (FP or FFP, respectively) (at least 30–50 ml/kg/day given initially at 10 ml/kg/h and then at 2 ml/kg/h). This is done to replace the consumed coagulation factors. Alternatively, fresh whole blood can be administered as a source for the coagulation factors, inhibitors as well as platelets. FP/FFP administration is aimed at halting the consumption of platelets, coagulation factors, and inhibitors (e.g., AT, a2-macroglobulin) in order to arrest the ongoing hemorrhagic and coagulation processes.
Heparin - Unfractionated and Low Molecular Weight Heparin
Administration of heparin, only during the peracute hypercoagulable phase, when PT and aPTT are shortened and AT III activity is at least 80%. Heparin can also be administered following FP/FFP administration and only when the laboratory coagulation tests were normalized.
The literature lacks controlled studies on the use of heparin in DIC. Clinical reports and retrospective studies do not provide a clear-cut indication whether heparin use is beneficial. Heparin effects may be variable, depending on the underlying cause and the stage of DIC.8 Various studies have shown either positive effects, no effect, or negative effects of heparin treatment and, therefore, its use in DIC is still under debate.
Heparin enhances thrombin and factor Xa inactivation through activation of AT III inhibitory actions and, therefore, is ineffective when AT III plasma activity is insufficient. Because AT III activity in DIC is usually low (as a result of consumption and possibly due to inactivation), it is advisable to provide the patient with sufficient quantities of this anticoagulant, most efficiently through blood component replacement. In a single study in dogs with different coagulopathies, FFP therapy did not result in increased plasma AT III activity.15
Heparin has another anti-clotting activity, through induction of the release of low affinity, microvasculature glycosaminoglycan-bound tissue factor pathway inhibitor (TFPI) pools into the circulation. Enhancement of TFPI activity represents an upstream, even more specific anticoagulatory action compared to AT III, in cases where coagulation is triggered by bacterial lipopolysaccharides in sepsis.16
To the best of our knowledge, there are no controlled studies determining the appropriate heparin dose for DIC in veterinary patients, or even substantiating its use in this syndrome. Extrapolation from the human literature is difficult because human patients at risk for DIC are generally also at high risk for deep vein thrombosis (DVT). They are consequently usually treated with aggressive heparin for DVT risk. This is generally not an issue for our patients. Controlled studies are difficult to perform since DIC is not a primary disease, and populations with DIC vary widely in terms of manifestation and prognosis due to variability in the underlying disease. Several authors have, however, proposed that in DIC sodium heparin is given at dose of 50-100 IU/kg SQ q8h. This dose should be adjusted through monitoring of the aPTT and AT III activity, with the aim to prolong the aPTT by up to 30% above the upper reference interval in a hypercoagulable state or, when such value is achieved through replacement and supportive therapy.2
Low molecular weight heparin (LMWH) is composed of heparin fractions with molecular weights of 4000 to 8000 daltons. LMWH was found to be more advantageous than unfractionated heparin (UFH) in dampening the activated coagulation. In human patients with endotoxemia, it has been shown to significantly reduce mortality. In a double-blind, controlled study in human DIC patients, LMWH was more beneficial compared to UFH in decreasing bleeding complications.17
UFH binds to AT III, resulting in a conformation change of AT III that leads to greatly enhanced inhibition of many coagulation factors, such as thrombin, Xa, XIa, XIIa, and IXa. Unlike UFH, LMWH, due to its small molecular size, cannot simultaneously bind to both AT III and thrombin, and, therefore, inhibits thrombin to a lesser extent. However, when compared to UFH, LMWH has greater affinity to and enhanced inhibitory efficiency of factor Xa. LMWH also has a lesser tendency to bind to macrophages, plasma proteins, and platelets, accounting for its limited hepatic clearance, prolonged half-life and better bioavailability. In humans, LMWH has been found to have a 2–4-fold longer half-life than UFH, with greater bioavailability and more predictable anticoagulant effects.18 In addition, with LMWH, the likelihood of developing heparin induced thrombocytopenia is reduced compared to UFH.18,19
Antifibrinolytic Agents
A large body of evidence in human medicine supports the use of antifibrinolytic drugs for control of hemorrhage in a variety of clinical settings. Antifibrinolytic therapy is commonly used in human patients undergoing cardiovascular, pediatric and orthopedic surgery, dental procedures, or in cases of severe menstrual or postpartum bleeding, and mainly in major trauma. To date, antifibrinolytic drugs are clinically used in people for hemorrhage control, including ε-aminocaproic acid (EACA), tranexamic acid (TxA), and aprotinin. The lysine analogues, EACA and TA, inhibit plasminogen, and to a lesser extent, increase antiplasmin activity, resulting in decreased fibrinolysis, with TxA having a 10-fold activity compared to EACA. The results of the large CRASH-2 trial showed that the administration of TxA within the first three hours after hospital admission reduced mortality in trauma patients. Mortality rates were lowest among patients who received TxA within the first hour after hospital admission, and the authors concluded that TxA should be given as early as possible to bleeding trauma patients.20
References
1. Levi M, de Jonge E, van der Poll T, et al. Disseminated intravascular coagulation. Thromb Haemost. 1999;82:695–705.
2. Feldman BF, Kirby R, Caldin M. Recognition and treatment of disseminated intravascular coagulation. In: Bonagura JD, ed. Kirk’s Current Veterinary Therapy XIII: Small Animal Practice, 13th ed. Philadelphia, PA: W.B. Saunders Company; 2000:190–194.
3. Bruchim Y, Aroch I, Saragusty J, et al. Disseminated intravascular coagulation. Compend Contin Educ Vet. 2008;30:E3.
4. Wada H, Hasegawa K, Watanabe M. DIC: an update on diagnosis and treatment. [Rinsho ketsueki] The Japanese Journal of Clinical Hematology. 2017;58:523–529.
5. Bruchim Y, Ginsburg I, Segev G, et al. Serum histones as biomarkers of the severity of heatstroke in dogs. Cell Stress Chaperones. 2017;22:903–910.
6. Couto CG. Disseminated intravascular coagulation in dogs and cats. Vet Med. 1999;547–553.
7. Bateman SW, Mathews KA, Abrams-Ogg ACG. Disseminated intravascular coagulation in dogs: review of the literature. J Vet Emerg Crit Care. 1998;8:29–45.
8. Levi M, ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592.
9. Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth. 1992;69:307–313.
10. Grant HW, Hadley GP. Prediction of neonatal sepsis by thromboelastography. Pediatr Surg Int. 1997;12:289–292.
11. Kraft W. Thrombelastogram in healthy domestic cats and therapy of disseminated intravascular coagulation (DIC) in panleukopenia. Berl Munch Tieraerztl Wochenschr. 1973;86:394–396.
12. Otto CM, Rieser TM, Brooks MB, et al. Evidence of hypercoagulability in dogs with parvoviral enteritis. J Am Vet Med Assoc. 2000;217:1500–1504.
13. von Schaewen H. Thrombelastographic studies and thrombocyte count in dogs. Berl Munch Tieraerztl Wochenschr. 1971;84:324–328.
14. Franchini M, Manzato F. Update of the treatment of disseminated intravascular coagulation. Hematology. 2004;9:81–85.
15. Rozanski EA, Hughes D, Scotti M, et al. The effect of heparin and fresh frozen plasma on plasma antithrombin III activity, prothrombin time and activated partial and thromboplastin time in critically ill dogs. J Vet Emerg Crit Care. 2001;11:15–21.
16. Pernerstorfer T, Hollenstein U, Hansen J-B, et al. Heparin blunts endotoxin-induced coagulation activation. Circulation. 1999;100:2485–2490.
17. Sakuragawa N, Hasegawa H, Maki M, et al. Clinical evaluation of low-molecular-weight heparin (FR-860) on disseminated intravascular coagulation (DIC) - a multicenter co-operative double-blind trial in comparison with heparin. Thromb Res. 1993;72:475–500.
18. Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688–699.
19. Prandoni P, Siragusa S, Girolami B, et al. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106:3049–3054.
20. CRASH 2 collaborators, Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101, 1101 e1091–1092.