M. Scherk
Over the recent decade, there has been increased awareness of pain and attention to the alleviation of pain in cats. Investigation has focused primarily on chronic musculoskeletal pain. The purpose of this presentation is to address other types of chronic and neuropathic pain in cats. For an excellent review of all types of pain, the reader is referred to the WSAVA Guidelines for recognition, assessment, and treatment of pain.1
Introduction
Pain isn’t just about how it feels; it is also about how it makes you feel. It results in suffering and a feeling of hopelessness. According to the International Association for the Study of Pain, pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.
Pain may be nociceptive, associated with injury (thermal, chemical, or traumatic/surgical), inflammatory, or neuropathic. There is a lot of overlap, and, regardless of type, if adequately controlled and primary or secondary inflammation resolve, pain should not become irreversible.
Acute pain is associated with tissue damage and serves to change behaviour in order to minimize or avoid damage. It is beneficial in that it helps to optimize conditions in which healing can take place. It is self-limiting and stops once healing is complete. Chronic pain, on the other hand, persists beyond the expected healing process without a clear end-point. It is maladaptive and dysfunctional and does not support healing. It can have significant effect on physical wellbeing and psychology of the sufferer. Chronic pain may be considered a disease state. Chronic pain may be present as a result of ongoing medical inflammation (e.g., intestinal, lower urinary tract, oral/dental, musculoskeletal) or secondary to unrelenting nociceptive stimulation (injury and associated inflammation).
Neuropathic pain is a term that refers to pain that is directly caused, or instigated, by dysfunction of, injury to, or primary lesion in the nervous system. As damaged nerves fire spontaneously, they become hyper- responsive to even normal stimuli. This pathophysiology results from sequential changes occurring in the peripheral nervous system, spinal cord, brainstem, and brain.1
Neuropathic pain is often a result of surgery, especially amputations (e.g., tail, limb, onychectomy) or fractures when appropriate analgesic agents have not been used or used for a long enough duration. Nerve compression and diabetes also result in neuropathic pain as can any chronic, unrelenting pain regardless of cause.2 Neoplasia, and probably interstitial/sterile idiopathic cystitis, are considered to be “mixed” as they have both inflammatory and neuropathic characteristics.
Understanding Pain
Tissue damage stimulates the nociceptors; this results in transduction (i.e., translation of the stimulus), transmission of the signal to the spinal cord where it is modulated (amplified or dampened) and then transmitted to the brain where the original stimulus is ultimately perceived (in the frontal cortex and limbic system). Therefore, it is likely that emotional and psychological elements play a role in cats as they do in people.3
Acute pain must be treated until inflammation is sufficiently resolved that the pain pathway won’t be aggravated anew. All patients need to be sent home with analgesic medication postoperatively, regardless of how “routine” the procedure is.4-6 If inadequate analgesia was provided following surgery or other trauma, or wasn’t administered for long enough, permanent changes to the central nervous system may occur resulting in the patient experiencing excessive and inappropriate pain. Persistent nociceptive input results in “wind-up,” an increase in the excitability of the sensory neurons of the spinal cord. These hyperexcitable cells amplify the signal that is sent to the brain resulting in changes to receptors and a decrease in inhibitory signals descending from the brain. Thus, the patient has a lower threshold to pain, experiencing it at a lower intensity than is expected (“allodynia”), has a greater pain response, experiencing more pain than expected for a given stimulus (“hyperalgesia”), and may have pain over wider regions than expected. In other words, it is important, not only to provide pain relief, but to provide it for a long enough period.3 (See Figure 1.)
| Figure 1 | 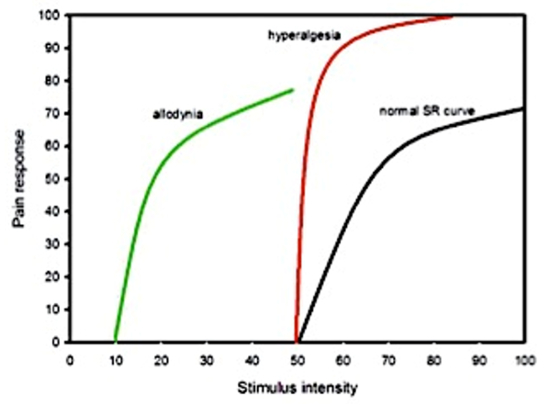
Allodynia, hyperalgesia vs. normal stimulus response curve. Image excerpted from Bergadano3 |
|
| |
Pain as an experience differs for each individual. Observe and adjust doses to make every patient comfortable.
Chronic Pain: Especially Older Cats but in Any Age
Oral diseases such as periodontal disease, root exposure, resorptive lesions, stomatitis, and oral ulcers and masses are all painful. Bacterial cystitis and pyelonephritis are more frequent in older cats but the prevalence of interstitial/sterile cystitis or inflammatory bowel disease does not differ from younger cats; inadequately addressed, these may cause on-going pain. The likelihood of neoplasia increases with increasing age. The need for analgesia must be considered as part of any treatment plan for the older cat. “Routine” procedures including blood collection, intravenous catheter placement, restraint of a thin or arthritic patient are uncomfortable.
Recognition of chronic pain and arthritic pain is relatively recent. The incidence of degenerative joint disease (DJD) appears to be much more common than previously thought and is probably a major cause of discomfort in ageing cats. In three studies retrospectively assessing radiographs taken of cats over 12 years of age7 or of any age8,9 the prevalence of findings suggestive of DJD was 90%, 22%, and 34%, respectively with older cats showing radiographic changes. Only 4%, 33%, and 16.5% had notation of restricted mobility in the medical record indicating that appropriate questions were not being asked of owners, that cats do not experience or that they don’t show discomfort from these joint changes.
A recent study10 prospectively evaluated cats of all ages to determine the prevalence of radiographic signs of DJD. Most (92%) cats had radiographic evidence of DJD; 91% had at least 1 appendicular site affected and 55% had >1 site of axial DJD. Affected joints in descending order of frequency were hip, stifle, tarsus, and elbow. The thoracic segment of the spine was more frequently affected than the lumbosacral segment. Grading the severity of each of the radiographic changes identified, they found that for every 1-year increase in age, the expected total DJD score increased by an estimated 13.6%. They concluded that radiographically visible DJD is very common in domesticated cats, even in the young and is strongly associated with age.
Yet lameness is not a common clinical sign of this problem in cats: signs are insidious or often attributed to ageing. They include inappropriate elimination (often adjacent to the litter box), decreased grooming, developing antipathy for being combed, reluctance to jump up or down, sleeping more, moving less, withdrawing from human interaction, and possibly even hiding. When activity monitors were attached to cats’ collars, activity counts increased with meloxicam suggesting alleviation of musculoskeletal discomfort.11
Wherever possible, the underlying cause of the pain should be identified and corrected.
Identifying Chronic Pain
Because cats are solitary survivors, they are notoriously secretive in revealing discomfort and disabilities. When the presenting concerns from the client fail to include observations of pain, questions regarding behavioural or life-style changes may elicit clues. Changes in awareness, personality, and interaction, an inappropriate activity level, reduction in playing, aggression, changes in sleeping patterns, and litter box use may be present. Other indicators of on-going pain include a decrease in mobility or ease of jumping (up or down), inappetence or altered eating behaviours and a poor coat from lack of grooming.12 Adults and older individuals are generally more stoic making it even harder to detect pain than in the kitten. Seriously ill or obtunded patients are especially difficult to assess for pain as they are less likely to display behavioural signs of distress when compared to an otherwise healthy injured cat.13
Examination may reveal reluctance to being handled or having a particular body part palpated or manipulated and may result in self-defensive behaviour. Sedation/anaesthesia may be needed to properly assess oral and dental problems or for imaging. Radiographic, ultrasonographic, or advanced imaging (MRI/CT) may be warranted to identify the underlying problem. Quantitated sensory testing may be undertaken to help localize the neuropathic lesion using different types of stimuli to identify the type (and therefore location) of nerve fiber affected.2
Elimination trials may be undertaken to verify and alleviate pain. For example, a local block may be used to assess oral/dental problems or a regional block for a joint or paw. An analgesic trial, usually based around opioids with or without non-steroidal anti-inflammatory drugs (NSAIDs) should also be considered when there is a suspicion of pain. The truest assessment of the presence of pain is response to analgesics resulting in return to normal behaviours.12
There are no pathognomonic or unique clinical signs that characterize pain or that are present in every painful individual. Cats cannot directly communicate their discomfort to us. Several pain scoring systems exist, however, they are either for assessing acute pain or have not been validated. The feline musculoskeletal pain index14 is the exception and may be a starting point for non-musculoskeletal pain. Fear (especially in the clinic) may look like pain and some patients may be experiencing both. With patients and in a quiet, calm environment, it can be easier to identify signs of pain. Look for at least three indicative signs in the history, behavioural changes, and examination.3 (See Figure 2).
| Figure 2 | 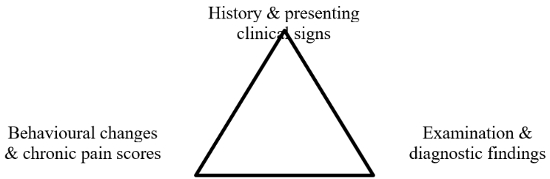
Triangulation for identifying chronic pain. Adapted from Bergadano3 |
|
| |
The experience of pain is different for every individual, both in severity, duration, and impact. An analgesic regimen, using single or multiple agents, needs to be tailored to the individual’s needs through empathic and repeated assessment.
Preventing and Treating Chronic Pain
Wherever possible, pre-emptive analgesia should be used to prevent stimulation of nociceptors and transduction of pain. Central sensitization can be prevented at the level of the N-methyl-D-aspartate (NMDA) receptors in the dorsal horn of the spinal cord. Ketamine is used, not only for its properties as a dissociative analgesic agent, but also specifically to block these receptors, thereby acting as an analgesic and as an anti-hyperalgesic agent.
When pre-existing inflammation, inadequate peri-operative analgesia with resultant neuropathic pain exist, or if the cause of pain cannot be treated, then an effective analgesic protocol must be developed in order to provide the patient with the best quality of life possible. Providing multimodal, balanced analgesia impacts multiple sites of the pain pathway while reducing the risk of negative effects from any one class of drug. This may be achieved through the concurrent use of an opioid with an NSAID and possibly amantadine (NMDA receptor antagonist) for maladaptive pain. Analgesic choices and doses for cats are listed in Table 1.
Table 1. Analgesic choices for chronic pain in cats
|
Drug class
|
Drug
|
Follow-up analgesia or chronic pain mild-moderate pain
|
Follow-up analgesia or chronic pain moderate-severe pain
|
|
Opioids
|
Butorphanol
|
0.1–0.4 mg/kg IV, IM, SC q2h
|
|
|
|
Buprenorphine
|
0.01–0.03 mg/kg IM, IV, SC q6–8h; 0.01–0.03 mg/kg q6–8h buccally
|
|
|
|
Morphine*
|
|
0.1–0.2 mg/kg IV q1–4h or 0.1–0.5 mg/kg IM q2–6h
|
|
|
Hydromorphone**
|
|
0.08–0.3 mg/kg IV, IM, q2–6h
|
|
|
Fentanyl
|
|
0.004–0.01 mg/kg IV q20m or 0.001–0.004 mg/kg/h CRI or fentanyl patch 12.5–25 mcg/h q4–5d
|
|
Opioid reversal or titration
|
Naloxone: for reversal/titration of opioid dose
|
Dilute 0.1 ml of 0.4 mg/ml naloxone in 5 ml 0.9% NaCl administer at 1.0 ml/minute to effect
|
Same
|
|
NSAIDs***
|
Meloxicam
|
0.1 mg/kg PO on d1, then 0.05 mg/kg PO q24h long-term, consider titrating to lowest effective daily dose (off-label)
|
Same
|
|
|
Robenacoxib
|
1–2 mg/kg PO q24h for 3 days (US, Canada), 6 days (EU)
|
Same
|
|
|
Ketoprofen
|
≤2.0 mg/kg SC once, then <1.0 mg.kg q24h for 4 days maximum
|
Same
|
|
|
Tolfenamic acid
|
≤4.0 mg/kg SC, PO q24h, for 3–5d
|
Same
|
|
NMDA receptor antagonists
|
Ketamine
|
0.5 mg/kg IV prn (q30m)
|
0.1–0.5 mg/kg/h IV CRI combined with morphine
|
|
|
Amantadine
|
|
3–5 mg/kg PO q24h for neuropathic pain
|
|
Sedatives for chronic pain of various levels in combination with opioids (not analgesics, provide relaxation)
|
Midazolam
|
0.1–0.5 mg/kg IV, IM q8–12h
|
Same
|
|
|
Diazepam
|
0.1–0.5 mg/kg IV, IM q12h
|
Same
|
|
|
Medetomidine
|
0.02–0.05 mg/kg IM q4–6h or 0.01–0.02 mg/kg IV prn
|
Same
|
|
Tricyclic antidepressant
|
Amitriptyline
|
2.5–12.5 mg/cat PO q25h
|
|
|
|
Gabapentin****
|
5–10 mg/kg PO q12–24h for mild-moderate postop, follow-up, chronic or neuropathic pain
|
|
*Morphine: Pretreat with Benadryl if administering IV.
**Hydromorphone: Caution: doses of 0.1 mg/kg and higher can cause hyperthermia in some patients; severe hyperthermia is an indication to change analgesic class.
***Meloxicam: The author and all cited studies are referring to Metacam® throughout this article. Compounded formulations lack third party, mandated and assessed quality control (QC); there is known potency variability between compounders and even between batches from the same compounder.
****Gabapentin: Taper dose when withdrawing drug. Do not discontinue abruptly.
Analgesia for Chronic Musculoskeletal Disease
The cat with joint pain is often an older patient who may have concurrent problems (e.g., renal disease) including some that may affect drug metabolism.15 Like painful patients of any age, they may be in a physiologic state that affects drug disposition, the most common ones being dehydration, inadequate tissue oxygenation, electrolyte or acid-base imbalances, and malnutrition. The most common concern regarding NSAID side effects is the possible consequence of using this class of drug in a dehydrated patient resulting in effects on gastric mucosal health or on renal function.16 Dehydration may be subclinical and difficult to assess in the very young and in the older cat due to the unreliability of skin elasticity in these age groups. (Stool consistency [i.e., pellets rather than formed logs] can be helpful in evaluating hydration.)
Opioids are safe for pain relief in any age group and are excellent when used at the same time as other agents, especially NSAIDs. They are not, however, a first drug of choice for cats with arthritic pain as they are not very effective for DJD. This is not to suggest that they shouldn’t be used for “break-through” pain or for comfort during diagnostic testing. If they produce adverse side- effects (e.g., euphoria, constipation, and inappetence) in an individual patient they may be reserved for palliative hospice care.
Pharmacokinetic data is lacking for safe, long-term use of many NSAIDs in cats. Carprofen half-life varies from nine to over 40 hours in cats.17,18 As most NSAIDs have long half-lives in cats when compared to other species, one precaution to avoid toxicity is to reduce the frequency of administration. Interestingly, despite having a short half-life of under 2 hours in blood, robenacoxib (Onsior®) its effect persists for 24 hours in clinical studies.
Metacam® 0.5 mg/ml oral suspension has been granted a license in the EU for the alleviation of inflammation and pain in chronic musculoskeletal disorders in cats. The registered dose is 0.1 mg/kg on the first day followed by 0.05 mg/kg orally once daily. This is the first NSAID licensed for long-term use in cats.
Numerous efficacy studies have been performed regarding both of these NSAIDs. In one, clients felt that cats treated for one month with meloxicam were more willing to jump achieving progressively higher heights during the study. Evaluation of the cats by the veterinarian at the end of the month showed a significant reduction of gait stiffness.19 Three studies have evaluated long-term safety of this agent in older cats; one concluded that this agent is safe, efficacious and palatable for musculoskeletal pain at 0.01–0.03 mg/kg PO q24h for a mean treatment duration of 5.8 months; no deleterious effect on renal function was detected in cats studied. Gastrointestinal upset in 4% of cats was the only adverse effect noted.20 The second and third, reviewed the medical records of cats over 7 years of age treated for a minimum of 6 months with a daily maintenance dose of 0.02 mg/kg meloxicam and concluded that this dose does not hasten progression of renal disease in aged cats or aged cats with pre-existent stable IRIS stage 1–3 renal disease.21,22
In 2015, a paper reported on the safety of robenacoxib (1–2.4 mg/kg) for daily, month long treatment of DJD in cats including 40 with chronic kidney disease IRIS stages 2–4. There was no evidence of increased risk in the frequency of reported adverse events, or in deterioration in renal variables in the subgroup of cats with concurrent CKD.23 Despite being similar to meloxicam (class, mechanism of action), by September 2016 it was licensed only for short-term use.
Excretion and metabolism of meloxicam have been studied in cats. After oral administration, the major route of excretion is fecal and the main pathway of biotransformation is by oxidation, rather than by their limited glucuronidation pathway. Additionally, 21% of the recovered drug was eliminated in urine (2% as unchanged meloxicam, 19% as metabolites) and 79% in the feces (49% as unchanged meloxicam, 30% as metabolites).24
A comprehensive review of the long-term use of NSAIDs in cats was published in 2010. This document may be accessed free-of-charge at: www.catvets.com/guidelines/practice-guidelines/nsaids-in-cats in Spanish, French, German, and Japanese.25 In addition, an educational client brochure (Spanish, French) regarding the safe use of NSAIDs in cats is also available at the same web link.
To minimize the risks of NSAIDs, it is important to:
- Select appropriate patients: individuals should maintain hydration and not be hypovolemic, hypotensive, or in congestive heart failure.
- Obtain a complete list of medications the cat is receiving or has access to.
- Base the dose on lean body weight and consider titrating, once pain is controlled, to lowest daily dose that maintains comfort.
- Use a balanced approach: include nutritional, adjunctive, and environmental components.
- Use gastroprotectants to treat or prevent gastric upset.
- Ensure communication with clients through verbal and written instructions.
- Recognize adverse reactions promptly and discontinue the NSAID.
- Monitor blood work q2–4 months (high risk patients) or q6 months (low risk patients25.
- A washout period of 3–5 days should be used if transitioning from one NSAID to another; a longer washout period is indicated (7–10 days or longer) when switching to, or from, aspirin or a corticosteroid. Additional, alternate analgesic agent(s) should be used during the washout period.25,26
A suitable protocol for a cat with pain from musculoskeletal disease might be baseline NSAID with intermittent use of an opioid (such as buprenorphine) when “break-through” pain is evidenced by a decrease in appetite, mobility, or social interaction. Gabapentin may be added for ongoing care.
Environmental modifications: Regular nail trimming helps by maintaining proper joint relationships. Ramps and steps to favourite sleeping spots are helpful. Warm, soft, padded sleeping places for stiff, painful, possibly bony joints should be considered. Raising food and water bowls may help the cat with cervical vertebral changes. Adding a litter tray to reduce the distance between boxes may reduce accidents as well as encourage regular voiding and defecation. The rim of the tray mustn’t be too high, nor the opening into the box too small. It should be scooped several times a day to encourage use.
Feeding a diet that is supplemented with eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) +/- green-lipped mussel (GLM) extract and glucosamine/chondroitin sulfate may be beneficial. Disease-modifying agents such as polysulfated glycosaminoglycan, glucosamine, and chondroitin sulfate may improve joint health.27 Additional modalities (therapeutic exercise, acupuncture, cold laser therapy) while no scientific studies have been done to support efficacy, may also play a role in providing comfort for a cat with musculoskeletal discomfort.
The author recommends that for chronic administration of NSAIDs in cats, it is good clinical practice to use the lowest effective dose based on lean body weight, tapering to the lowest effective daily dose (off-label), and to avoiding use in, (or use lower initial doses) in cats with renal disease. Ensure that the patient is hydrated and give the NSAID with food. Individual patients respond differently to the same agent and dose. In most cases, NSAIDs are most effective when used in conjunction with other treatment modalities.
Analgesia for Neuropathic Pain
Neuropathic pain may be caused by inadequately alleviated traumatic or surgery-induced pain, such as onychectomy (declaw) or amputation.28 Clients may remark that their cat doesn’t jump as high as before the procedure or walks as if on glass or eggshells. Alternately, they may note decreased activity, increased aggression, inappetence starting months or even years after surgery. While the initiating event may be known, it is imperative that radiographs of affected paws be taken to rule out a bone remnant, a surgically treatable problem. When none is found, neuropathic pain is treated by addressing “wind-up” while concurrently providing analgesia. Analgesics alone are ineffective. Amantadine is used off-label to block the NMDA receptors in the spinal cord, however, because it lacks analgesic effects, an opioid plus NSAID are used concurrently. The regime suggested by Gaynor is outlined in Table 2.
Table 2. Off-label protocol for alleviating neuropathic pain from onychectomy (Gaynor)24
|
Administer concurrently: Amantadine 3 mg/kg PO q24h x 21 days
|
|
+ Buprenorphine 0.01–0.02 mg/kg buccally q12h x 2–3 days
|
|
+ Meloxicam starting at 0.05 mg/kg PO q24h x 4 days => tapering to 0.05 mg/2 kg PO q24h x 4 days => followed by 0.05 mg/cat PO q24h x 4 days and finally => 0.05 mg/cat PO q48h x 5 days†
|
|
†The ISFM and AAFP consensus guidelines: Long-term use of NSAIDs in cats21 recommends once daily dosing rather than other frequencies.
|
Another condition, feline orofacial pain syndrome (FOPS) is a disorder of cats with behavioural signs of oral discomfort and tongue mutilation. There is suggestion that it is inherited in an autosomal recessive manner. It is believed to be neuropathic in nature and characteristically includes exaggerated licking and chewing movements, and pawing at the mouth; in some extreme cases mutilation of tongue, lips, and buccal mucosa occurs. It appears to be triggered by mouth movements (grooming, eating).29 Like idiopathic cystitis, it occurs at irregular intervals with the cat appearing to be pain-free in between these episodes. In both, external factors can also influence the disease such as anything causing stress or anxiety as well as other illness.29,30
Therapy for FOPS includes ruling out other causes of facial and oral pain, any dental disease discovered should be treated. An attempt to identify and eliminate environmental stresses and triggers should be made. Pain relief requires multiple agents including NSAIDs plus phenobarbital, carbamazepine, gabapentin, or amitriptyline. Treatment is long-term and may not be successful in some cases.31
Novel Mediators of Pain
New mediators of pain have been identified and are being studied as therapeutic targets. These include nerve growth factor (NGF), piprants, neurokinin-1 antagonists, selective neurotoxins, and cannabinoids.
- Nerve growth factor
This mediator of inflammatory and neuropathic pain is elevated in models of chronic and animal pain.32 Hyperalgesia is alleviated by inhibition of NGF. Numerous approaches are being evaluated, (e.g., monoclonal antibodies) to negate its effect. Risks and benefits of Tanezumab have been studied in human medicine for interstitial cystitis, osteoarthritis, diabetic neuropathy, and post-herpetic neuralgia. A felinized anti- NGF monoclonal antibody (NV-02, Frunevetmab, Nexvet Biopharma) has been developed; multicenter clinical trials are underway.33-34
- Piprants
Piprants and substances that antagonize prostaglandin E2 EP4 receptor, i.e., further down the inflammatory cascade than NSAIDs thereby not interfering with the “housekeeping” actions of COX enzymes. While not yet approved for use in cats, grapiprant (Galliprant®, Elanco) has been studied in this species and has received FDA approval for use in dogs with DJD.35
- Neurokinin-1 antagonists
This class of drug prevents substance P from binding to NK-1 receptors. Maropitant is typically used as an antiemetic but appears to provide visceral analgesia in dogs as indicated by reduced anaesthetic requirements during ovariohysterectomy.
- Selective neurotoxins
Two selective neurotoxins have been studied in dogs: resiniferatoxin and substance P-saporin. These selectively inhibit or destroy cells in receptors. It is too early to say whether these will play a role in veterinary analgesia.
- Cannabinoids
The use and benefits of medical marijuana for people continue to be investigated. Cannabinoids interact with receptors in the endocannabinoid system. These include cannabidiol (CBD), cannabinol (CBN), and tetrahydrocannabinol (THC) receptors. Fractions that target CBD and CBN receptors (e.g., as in hemp oil) but not THC receptors might be of use; THC is dangerous for small animals. Research is lacking for use for cannabinoids in cats at this time.
Selected References
1. Mathews K, Kronen PW, Lascelles BDX et al. Guidelines for recognition, assessment and treatment of pain. J Small Anim Pract. 2014;55:E10–E68.
2. Robertson SA, Lascelles BDX. Long-term pain in cats: how much do we know about this important welfare issue? J Fel Med Surg. 2010;12:188–199.
3. Bergadano A. Diagnosis of chronic pain in small animals. Europ J Companion Anim Pract. 2010;20(1):55–60.
20. Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg. 2008;10:235–241.
22. Gowan RA, Baral RM, Lingard AE, et al. A retrospective analysis of the effects of meloxicam on the longevity of aged cats with and without overt chronic kidney disease. J Feline Med Surg. 2012;14(12):876–881.
23. King JN, King S, Budsberg SC, et al. Clinical safety of robenacoxib in feline osteoarthritis: results of a randomized, blinded, placebo-controlled clinical trial. J Feline Med Surg. 2015;1098612X15590870.
25. Sparkes AH, Helene R, Lascelles BCX et al. ISFM and AAFP consensus guidelines: Long-term use of NSAIDs in cats. J Fel Med Surg. 2010;12:521–538.
Complete references available from the author.