Standardization of Ultrasonographic Anatomy of Coelomic Organs in Indian Spectacled Cobra (Naja naja) and Russell’s Viper (Daboia russelii)
Abstract
There is scarcity of reports on ultrasonographic studies of the coelomic cavity in Indian spectacled cobra (Naja naja) and Russell viper (Daboia russelii). A total of ten adult Indian spectacled cobras and six adult Russell vipers were used for this study. Ultrasonography was performed using a Doppler ultrasound unit equipped with a probe of 6.0 to 10 MHz frequency. The snakes were restrained without anaesthesia, with help of a restraining tube and held in ventrodorsal recumbency by two assistants. A thick layer of acoustic coupling gel was applied ventrally to ensure optimal contact between the probe and the snake’s scales. Cardiac mode of the ultrasound was used for better visualisation of coelomic organs. The snakes were scanned across the ventral body wall methodically from a rostral to caudal direction using transverse and sagittal scanning planes to enable all organs to be identified. The liver was elongated; echogenicity of the liver was homogenous, and the vena cava appeared as a hyperechogenic line dividing the liver into two halves on transverse plane. A large central hepatic vein was evident on examination. Gall bladder appeared as an anechoic focal area well caudal to the liver. Spleen appeared as a small regular sphere slightly more hyperechoic than the liver. The colon appeared anechoic with hyperechoic floating urates. Echogenicity of the kidney was similar to that of liver, plurilobulated in nature. Such knowledge of the normal ultrasonographic anatomy of coelomic organs of individual species is important for accurate diagnosis and interpretation of routine health examinations.
Introduction
Abdominal ultrasonography is a noninvasive technique that can be used for diagnosis of abdominal disorders and pregnancy.17 The normal ultrasonographic anatomy of visceral organs in domestic small animal species and some wild mammals is well documented,3,4,11,13,16,18,19 but there are very few established reports on ultrasound study in reptiles.1,2,6,12,14,15,21-25 The Indian spectacled cobra is native to the Indian subcontinent which includes present-day Nepal, Pakistan, India, Bangladesh and Sri Lanka. Its distribution ranges from sea level up to 2,000 m (6,560 ft) above sea level.26 Russell’s viper is found in India, Pakistan, Sri Lanka, Bangladesh, Nepal and in some Southeast Asian countries like Myanmar and Thailand. Its distribution ranges from sea level up to 2,756 m (9040 ft) above sea level.27
Both the species are often found in highly urbanized areas and settlements in the countryside, the attraction being the rodents commensal with man. Because of this, they are commonly involved in human–animal conflict. Many cases are presented commonly to wildlife veterinarians and wildlife rehabilitation centers with resultant injuries from such conflicts (Krishna et al. 2013). Captive populations of both species are found in almost all the zoos in India and are also kept as exotic pets in many western countries. The aim of this present work was to study the normal echogenicity and location of visceral organs in the coelomic cavity of these two species so that it can be used as future reference. Such knowledge of the normal ultrasonographic anatomy of visceral organs of individual species is also important for accurate diagnosis and interpretation at routine health examinations.
Materials and Methods
The study was carried out in the Department of Veterinary Medicine, Veterinary College, Bengaluru, India. A total of ten adult Indian spectacled cobras and six adult Russell’s vipers were used for this study. All the snakes used for the study were rescued from populated areas in and around Bangalore. Necessary permissions were procured from Principal Chief Conservator of Forest (Wildlife), Karnataka Forest Department. Snakes selected for the study appeared to be healthy and clinically stable based on activity level, physical examinations, and body condition scores, although some had minor superficial wounds. Only those snakes weighing more than 500 g were selected for the study. Spectacled cobras used for study had average length of ±1.43 m, and Russell’s vipers had average length of ±0.97 m.
The ultrasonographic examination was done using a 6 to 10 MHz curvilinear array sector Doppler probe (GE Logiq Book XP). Each snake was restrained without anaesthesia with help of a restraining tube (Midwest®) and held in ventrodorsal recumbency by two assistants. Ventral scales were cleaned thoroughly with an alcohol swab, dried, and a thick layer of acoustic coupling gel was applied ventrally at least 5 minutes before application of the transducer to ensure optimal contact between the probe and the snake’s scales.24 Cardiac mode of the ultrasound was used for better visualisation of organs. The snakes were scanned across the ventral body wall methodically from a rostral to caudal direction using transverse and sagittal scanning planes to enable all organs to be identified. Apex of the heart was used as a landmark at the rostral end.15 The probe was then moved toward the cloacal opening, which was used as a landmark at the caudal end. Anatomical position and echogenicity of visible visceral organs were recorded and analyzed in each snake.
Results
The liver was visible in all the snakes. It was elongated and had a homogenous echotexture throughout its length. Echogenicity of the liver ranged from hypo- to hyperechoic, and the vena cava appeared as a hyperechogenic line dividing the liver into two halves on transverse plane (Figure 1). A large central hepatic vein, anechoic in nature, was evident on examination (Figure 2). Anatomically, the liver was located in the mid-third of the body, from apex of the heart, and ended well cranial to the gall bladder.
Gall bladder appeared as an anechoic focal area well caudal to the liver. It was visible in all the snakes (Figure 3). When the snake was anorectic for a period of time, the gall bladder was often greatly enlarged, filling approximately one-third of the diameter. Spleen, located close to gallbladder, appeared as a small regular sphere slightly more hyperechoic than the liver and was visible only in three specimens.
Colon appeared anechoic. It was visualized in snakes having digested prey, hyperechoic floating urate crystals, air or fluid (Figure 4). Anatomically, the colon was located in the caudal third of the body, close to the kidney. Evaluation of the kidney was done by identifying each kidney in the caudal third of the body, dorsolateral to the colon. Echogenicity of the kidney was similar to that of liver. The kidneys showed plurilobulation (Figure 5), with the right kidney larger and positioned anteriorly compared to the left. Both the kidneys were visualised in all the snakes. Ultrasonographically, detailed structures of kidneys were not distinguishable.
Testes could not be identified in any of the snakes. Although ovaries could not be identified in any of the snakes, one Russell’s viper (ovoviviparous species) showed skeletons and movements of embryos (Figure 6).
Discussion
The 6 to 10 MHz curvilinear array sector Doppler probe (GE Logiq Book XP) used for ultrasonographic examination could differentiate between echogenicity of different coelomic organs in both the species used for the study. Compared to other modes, cardiac mode of the ultrasound gave better view of organs. The use of copious amounts of acoustic coupling gel gave satisfactory results, with minimal artifacts.
Studies have demonstrated that the liver was the most evident structure among the viscera of the coelomic cavity in aquatic snakes.5 Earlier studies have also explained that serpents have single-lobed, cylindrical, tubular liver which begins just behind the heart and extends to the middle of the stomach.24 Observations in this present study also correlate with above studies. In all, the liver was located in the mid-third of the body, from the apex of the heart to a bit cranial to the gall bladder, covering a large area in the coelomic cavity.
Some of the authors in their ultrasonographic studies have described findings regarding the image of large vessels in snakes during ultrasound scanning.25 There is also an opinion that snakes have a typical large hepatic vein, visualized as an anechoic stripe that helps differentiate the liver from fat bodies.24 This study also confirmed the presence of two large vessels: caudal vena cava and hepatic portal vein. Vena cava appeared as a hyperechoic line dividing the liver into two halves on transverse plane. A large central hepatic vein, anechoic in nature, was evident on examination in all the snakes. This investigation also showed that it is possible to calculate portal vein velocity using standard 6- to 10-MHz probe.
The gall bladder in reticulated python, unlike in domestic animals, is not associated nor attached directly to the hepatic parenchyma. There may be difficulty in imaging of the gall bladder in reticulated pythons due to the obstruction brought about by the presence of numerous adipose tissues and/or ovarian follicles.1 In this present study the gall bladder appeared as an anechoic focal area well caudal to the liver which showed that it was not directly attached to liver. However, there was no difficulty in visualizing the gall bladder in both species. The reason behind this may be less accumulation of adipose tissue in both species in study compared to reticulated pythons or boids in general.
The majority of snake species have the right kidney positioned anteriorly compared to that of the left.8-10,20 Present studies also had similar findings, where the right kidney was larger and positioned anteriorly compared to the left kidney in both Indian spectacled cobra and Russell’s viper. Some studies have demonstrated that the kidney was plurilobulated in snakes.24 Certain studies have also explained the gross anatomy of the kidney in Indian spectacled cobra, demonstrating lobulations in it through postmortem examinations (Sridevu et al. 2013). This study also confirmed the presence of multiple lobes in each kidney in both the species.
Even though adult snakes were used for the study, testes could not be visualized in any of the snakes. Testes are slender and fusiform in shape, making them difficult to image in snakes.24 Difficulty in visualizing testes in this study can also be attributed to the relatively small size and slender shape of both the species.
According to some authors the inactive ovaries are anechogenic and small in snakes. Eggs, if present in the oviducts, appear as two distinguishable layers: a superficial layer formed of anechogenic albumen, and the deeper layer formed of more echogenic vitellus. Around the circumference of the eggs, eggshell is found whose echogenicity depends on the degree of calcification. In ovoviviparous species, skeletons and movements of embryos are easily visualized.12,14,15,24 In this study no such structures were visualized except for one Russell’s viper where movement of embryos was visualized and recorded.
Conclusions
Ultrasound examination can be used as a good diagnostic tool in reptile medicine to provide information regarding organ location, pathologic changes, pregnancy and confirmation of sex. The results of this study can serve as baseline information for the ultrasonographic examination of coelomic organs in Indian spectacled cobra and Russell’s viper in the future.
Table 1. Ultrasonographic measurement of liver thickness in spectacled cobra and Russell’s viper.
|
Species
|
Range (cm)
|
Mean±SD (cm)
|
|
Indian spectacled cobra (n=10)
|
0.69–1.72
|
1.101±0.11
|
|
Russell’s viper (n=6)
|
0.51–1.73
|
1.04±0.18
|
Figure 1. Homogenous liver and vena cava appearing as hyperechogenic line dividing the liver into equal halves on transverse plane in a spectacled cobra
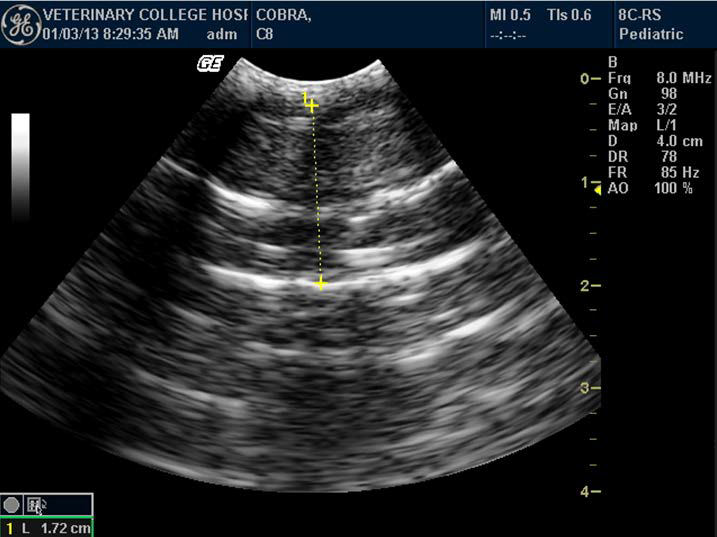
Figure 2. Portal vein and calculation of its velocity in Indian spectacled cobra
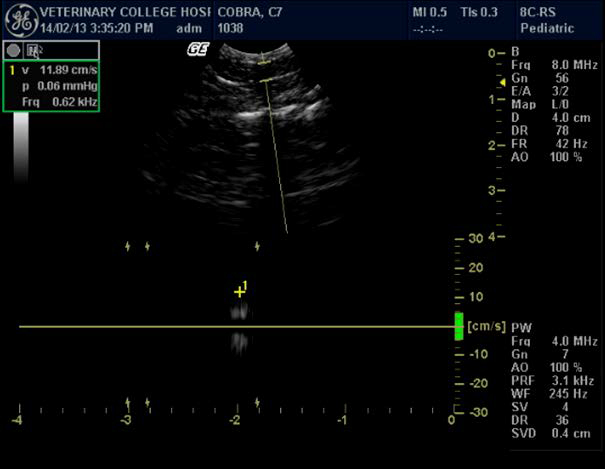
Figure 3. Enlarged gall bladder in Indian spectacled cobra
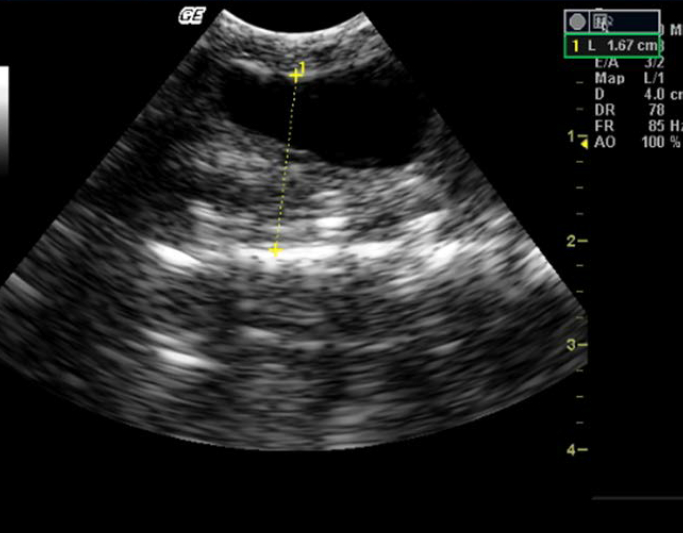
Figure 4. Anechoic colon of Russell’s viper with hyperechoic floating urates
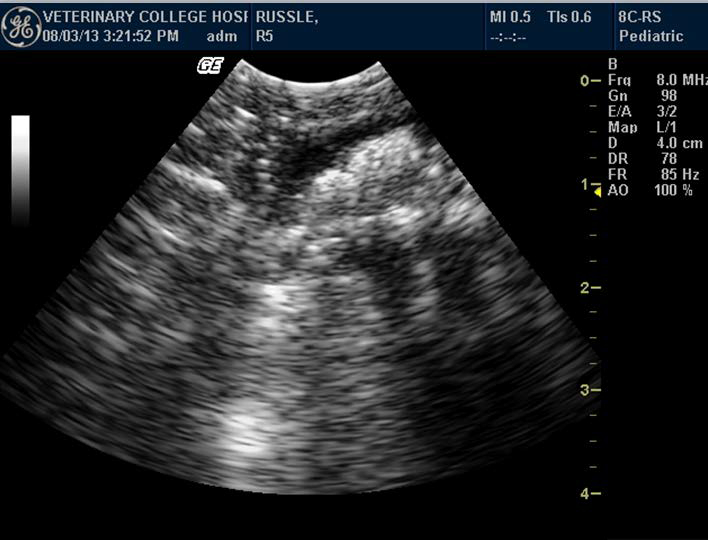
Figure 5. Appearance of normal kidney in Indian spectacled cobra
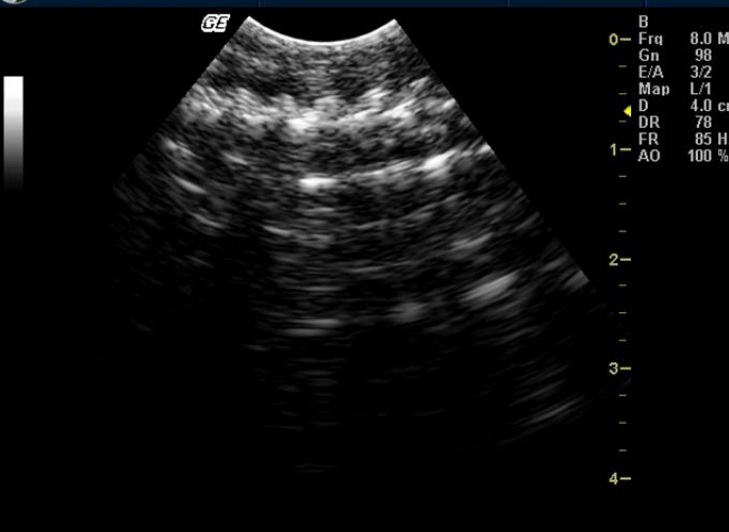
Figure 6. Skeletons and movements of embryos in Russell’s viper
Literature Cited
1. Aguisanda, S. T., E. A. Lastica, and J. A. Acorda. 2011. Ultrasonographic features of the liver, gall bladder and spleen of captive reticulated pythons (Python reticulatus). Philipp J Vet Anim Sci. 37:177–186.
2. Banzato, T. E., L. Russo, M.C. Finotti, M. Milan, M. Gianesella, and A. Zotti. 2012. Ultrasonographic anatomy of the coelomic organs of boid snakes (Boa constrictor imperator, Python regius, Python molurus molurus, and Python curtus). Am J Vet Res. 73:634–45.
3. Bapodra, P., P. Mahoney, S. Turner, A. Silva-Fletcher, and M Waters. 2010. Ultrasonographic anatomy of the Asian elephant (Elephas maximus) eye. J Zoo Wildl Med. 41:409–417.
4. Barr, F. J., P. E. Holt, and C. Gibbs. 1990. Ultrasonographic measurement of normal renal parameters. J Small Anim Pract. 31:180–184.
5. Bragdon, D. E., 1953a. A contribution to the surgical anatomy of the water snake, Natrix sipedon sipedon; the location of the visceral endocrine organs with reference to ventral scutellation. Anat Rec. 117:145–161.
6. Bragdon, D. E., 1953b. Lipid-distribution in corpora lutea of pregnancy in the water snake, Natrix sipedon-sipedon. Anat Rec. 115:288–288.
7. Cerveny, S. N. S., M. M. Garner, J. J. D’Agostino, S. R. Sekscienski, M. E. Payton, and M. R. Davis. 2012. Evaluation of gastroscopic biopsy for diagnosis of Cryptosporidium sp. Infection in snakes. J Zoo Wildl Med. 43:864–871.
8. Chiodini, R. J., J. P. Sundberg, and J. Czikowsky. 1981. Gross anatomy of the snake. Vet Med Sm Anim Clin. 77:413–419.
9. Divers, S. J., G. Ness, M. P. C. Lawton, and J. Wyneken. 1999. Surgical anatomy of serpentine (Colubridae and Boidae) kidney with particular regard to surgical nephrectomy. Proc Assoc Rept Amph Vet Annu Conf. Pp. 175–178.
10. Fox, H., 1977. The urogenital system of reptiles. In: Gans C, Parson T, eds. Biology of Reptilia. Academic Press, New York, NY: 1–157.
11. Geisse, A. L., J. E. Lowry, D. J. Schaeffer, and C. W. Smith. 1997. Sonographic evaluation of urinary bladder wall thickness in normal dogs. Vet Radiol Ultrasound. 38:132–137.
12. Hernandez-Divers, S., and S.H. Hernandez-Divers. 2001. Diagnostic imaging of reptiles. In: Practice, July/August: 370–390.
13. Hittmair, K. M., H. D. Vielgrader, and G. Loupal. 2001. Ultrasonographic evaluation of gall bladder wall thickness in cats. Vet Radiol Ultrasound. 42:149–155.
14. Hochleitner, C., and M. Hochleitner. 2004. Ultrasound in reptiles. Proc Assoc Rept Amph Vet. Naples, FL:41–44.
15. Isaza, R., N. Ackerman, and E.R. Jacobson. 1993. Ultrasound imaging of the coelomic structures in the boa constrictor (Boa constrictor). Vet Radiol. 34:445–450.
16. Kathleen, A. L., A. S. Templer, J. R. Paul-Murphy, R. T. O’Brien, B. K. Hartup, and J. A. Langenberg. 2003. Ultrasonographic imaging of the sandhill crane (Grus canadensis) intertarsal joint. J Zoo Wildl Med. 34:144–152.
17. Lamb, C. R. 1995. Abdominal ultrasonography in small animals. In: Goddard PJ, ed. Veterinary Ultrasonography. Univ. Press, Cambridge, United Kingdom: 21–54.
18. Makungu, M., W. M. du Plessis, M. Barrows, K. N. Koeppel, and H. B. Groenewald. 2012. Ultrasonographic abdominal anatomy of healthy captive caracals (Caracal caracal). J Zoo Wildl Med. 43:522–529.
19. Mattoon, J. S., D. M. Auld, and T. G. Nyland.2002. Abdominal ultrasound scanning techniques. In: Nyland, T. G. and J. S. Mattoon, eds. Small Animal Diagnostic Ultrasound. 2nd ed. W. B. Saunders Co.; Philadelphia, PA: 49–81.
20. McCraken, H.E. 1999. Organ location in snakes for diagnostic and surgical evaluation. In: Fowler, M.E., R.E. Miller, eds. Zoo and Wild Animal Medicine: Current Therapy. WB Saunders Co; Philadelphia, PA: 747.
21. Neto, F.C.P., P. C. War, F. B. Costa, A. Araujo, M. A. Miglino; P. P. Bombonato; L. C. Vulcan, and F. R. Alves. 2009. Ultrasonography of the liver, renal and reproductive apparatus of boa constrictor snake. Pesq Vet Bras. [online]. 29:317–321.
22. Pease, A., Gaële Blanvillain, D. Rostal, D. Owens, and A. Segars. 2010. Ultrasound imaging of the inguinal region of adult male loggerhead sea turtles (Caretta caretta). J. Zoo Wildl Med. 41:69–76.
23. Schilliger, L., D. Tessier. J. L. Pouchelon and V. Chetboul. 2006. Proposed standardization of the two-dimensional echocardiographic examination in snakes. J Herpetol Med Surg. 16:90–102.
24. Schilliger LH. 2010. Ultrasound examination in reptiles: restraint, positioning, artifacts and patient examination. Paris, France. http://clinvet-auteuil.com/IMG/pdf/LS-Ultrasound.pdf. (VIN editor: This link was not accessible as of 12-17-20.)
25. Snyder, P.S., N.G Shaw, and D.J. Heard. 1999. Two-dimensional echo-cardiographic anatomy of the snake heart (Python molurus bivittatus). Vet Radiol Ultrasound. 40:66–72.
26. Whitaker, R., and A. Captain. 2004a. Snakes of India: The Field Guide. Draco Books. Chennai, India. Pp. 304.
27. Whitaker, R., and A. Captain. 2004b. Snakes of India: The Field Guide. Draco Books. Chennai, India. Pp. 332.