Abstract
Pelvic limb abnormalities significantly reduce chances for survival in many avian species, and the long limbs
of cranes make them particularly vulnerable to injury.1-4 Fractures and joint luxations are relatively easy to diagnose
using radiography.5,6 However, injuries to tendons and ligaments can be difficult or impossible to diagnose and localize
with radiography alone. Surgical exploration is often necessary to define the location and severity of musculotendinous injury, but
extensive approaches can cause unacceptable morbidity. Precise, noninvasive definition of soft tissue injuries could direct
treatment and improve chances for full recovery.
Real-time ultrasonographic imaging is a useful modality for diagnosing musculoskeletal injuries in
mammals.7-11 Its use has been reported rarely in birds, but in one study it was found to provide a ready means for
postmortem diagnosis of osteomyelitis in turkeys.12 Anatomic studies have been limited to a brief report on whole body
imaging of an emu.13
Our study aimed to assess the effectiveness of this modality at defining soft tissue structures within the
intratarsal joints of sandhill cranes (Grus canadensis). Ultrasonographic imaging was first correlated with
topographic14-15 and cross-sectional anatomy of two cadaver limbs. These findings were then compared with results of
ultrasonographic examination of the intratarsal joints of five living, normal adult sandhill cranes. In addition, the intratarsal
joints of three cranes with known abnormalities were imaged and compared with normal joints to explore the ability of
ultrasonography to detect aberrations within soft tissue structures. All imaging was performed with a GE Logic Ultrasound unit with
a linear transducer set at 10 MHz. The cranes we examined were habituated to being handled by people, and they were anesthetized
with isoflurane while being imaged.
The paucity of overlying soft tissue prevented useful imaging of the medial and lateral aspects of the
intratarsal joint. It was also difficult to image tendons proximal and distal to this joint because the mineralization present in
virtually every tendon caused considerable noise in the signals the transducer received. However, it was possible to see major
tendons, retinacula and blood vessels as they traversed the cranial and caudal aspects of the joint. Structures consistently
visible on the dorsal aspect of the intratarsal joint included the cranial tibial tendon (including its point of insertion) and the
cranial tibial artery (Figures 1, 2). Longitudinal imaging of the caudal aspect of the intratarsal joint revealed four tiers of soft
tissue. The most superficial of these was the gastrocnemius tendon, followed in order of increasing depth by the flexor perforans
et perforati digiti III, combined tendons of the flexor perforans et perforati digiti II and flexor perforatus digiti IV, and
finally the deep digital flexor tendon (Figure 3). The tibial cartilage was not obvious on the longitudinal view, but it was very
evident on transverse section as it served to separate the tendons coursing through it. In the transverse view of the caudal aspect
of the intratarsal joint the gastrocnemius tendon was clearly imaged along with the retinaculum which binds this tendon to the
tibiotarsus. The digital flexor tendons listed above appeared as discreet hypoechoic structures with hyperechoic rims within the
homogeneous tibial cartilage (Figure 4).
Imaging of cranes with known tarsal abnormalities revealed changes from our reference group in each case. A
Siberian crane with intratarsal joint swelling secondary to an angular limb deformity had a marked increase in medial retinacular
thickness on the transverse view of the caudal aspect of the intratarsal joint. Ultrasonography of another Siberian crane with a
gastrocnemius tendon that could be manually luxated to the medial side of the intratarsal joint revealed effusion dorsal to the
tibial cartilage, displacement of the tibial cartilage and apparent disruption of continuity of the gastrocnemius and flexor
tendons. Finally, imaging of a sandhill crane with soft tissue swelling cranial to the intratarsal joint revealed an avulsion of
the insertion of the cranial tibial tendon with retraction of the tendon to a point 3 cm proximal to the joint. These findings were
confirmed during surgical exploration of the area.
Ultrasonographic imaging can be used to evaluate some-but not all-of the soft tissue structures around the
intratarsal joint of cranes and may be a useful adjunct to physical and radiographic examination of injuries in this area. Its
greatest value may be in localizing the injury so that treatment can be specific and refined.
Click on an image to see a larger view
| Figure 1. | 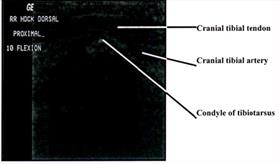
Longitudinal image of the cranial aspect of the intratarsal joint. Proximal is to the left and cranial is to the top of this figure. |
|
| |
| | Figure 2. | 
Transverse image of the cranial aspect of the intratarsal joint. Cranial is to the top and lateral is to the left of this figure. |
|
| |
|
| Figure 3. | 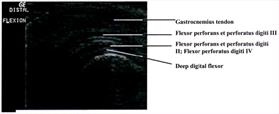
Longitudinal image of the caudal aspect of the intratarsal joint. Caudal is to the top and distal is to the left on this image. |
|
| |
| | Figure 4. | 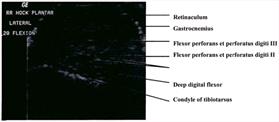
Transverse image of the caudal aspect of the intratarsal joint. Caudal is to the top and lateral is to the left on this image. |
|
| |
|
Acknowledgments
We would like to thank the folks at International Crane Foundation for their collaboration on this project.
Funding for this study was provided by the Companion Animal Fund of the School of Veterinary Medicine at University of
Wisconsin-Madison.
References
1. Bush M. 1977. External fixation of avian fractures. J. Am. Anim. Hosp. Assoc. 171: 943.
2. Lind PJ, Gushwa DA, VanEk JA. 1988. Fracture repair in two owls using polypropylene rods and
acrylic bone cement. Assoc. Avian Vet. Today. 2: 128.
3. MacCoy DM. 1983. High density polymer rods as an intramedullary fixation device in birds. J.
Am. Anim. Hosp. Assoc. 19: 767.
4. Smith SA, Smith BJ. 1990. Normal xeroradiographic and radiographic anatomy of the red-tailed hawk
(Buteo jamaicensis), with reference to other diurnal raptors. Vet. Radiol. 31: 301.
5. Curro TG, Langenberg JA, Deakin L. 1996. Radiographic analysis of the development of the pelvic
limb of captive-reared cranes (Grus spp.). Zoo. Biol. 15: 143.
6. Bush M, Montali RJ, Novak GR, James AE. 1976. The healing of avian fractures: a histological
xeroradiographic study. J. Am. Anim. Hosp. Assoc. 12: 768.
7. Middleton WD, Reinus WR, Totty WG, Melson GL, Murphy WA. 1985. US of the biceps tendon apparatus.
Radiology 157: 211.
8. Lind T, Reimann I, Larsen JK, Karstrups S. 1989. Sonography in soft-tissue trauma of the
shoulder. Acta Orthop. Scand. 60: 49.
9. Bohn A, Papageorges M, Grant BA. 1992. Ultrasonographic evaluation and surgical treatment of
humeral osteitis and bicipital tenosynovitis in a horse. J. Am. Vet. Med. Assoc. 201: 305.
10. Redding WR. 1993. Evaluation of the equine digital flexor tendon sheath using diagnostic ultrasound and
contrast radiography. Vet. Radiol. Ultrasound. 34: 42.
11. Hauser ML, Rantanen NW. 1983. Ultrasound appearance of the palmar metacarpal soft tissue of the horse.
J. Eq. Vet. Science. 3:19.
12. Mutalib A, Holland M, Barnes HJ, Boyle C. 1996. Ultrasound for detecting osteomyelitis in turkeys.
Avian Diseases. 40: 321-325.
13. Tully TN, Hillman D, Williams J. 1995. Anatomic examination of an emu (Dromauis novaehollandiae)
using ultrasonographic imaging: techniques and anatomic cross sections (abstract). Proc. Assoc. Avian Vet. 115: 313.
14. Fisher HI, Goodman DC. 1955. The myology of the whooping crane, Grus americana. Illinois
Biological Monographs. 24: 76-102.
15. Orosz SE, Ensley PK, Haynes CJ. 1992. Avian Surgical Anatomy. W.B. Saunders Company,
Philadelphia, Pennsylvania, Pp. 90-97.