Abstract
Blood gas and acid base measurements are an essential means of assessing respiratory function and
homeostasis. Blood levels of oxygen and carbon dioxide are closely related to and affected by acid base balance and
electrolyte concentrations. This delicate balance can be altered by minute changes in respiratory patterns, body
temperature, and metabolic demands. If unrecognized, severe consequences may result including neurologic or myocardial
dysfunction and multiorgan failure. Blood gas evaluation has been reported for only a few nondomestic species.
Studies to evaluate blood gas parameters in bongo (Tragelaphus eurycerus) and eland
(Tragelaphus oryx) antelope were conducted at The Audubon Center for Research of Endangered Species, the Audubon
Park Zoo, and the Mt. Kenya Game Ranch. Thirteen adult female captive bongo, 11 adult female captive eland and six adult
female free-ranging eland were bled under three different circumstances: 1) Manual restraint in a drop floor chute (Fauna
Research Inc. Red Hook, NY 12571 USA). 2) Manual restraint following sedation. Animals received haloperidol (Geneva
Pharmaceuticals Inc. Broomfield, CO 80020 USA) at an approximate dose of 1 mg/kg per os s.i.d. for 3 days prior to
sampling and 3 hr prior to restraint on the day of sampling. 3) Chemical immobilization using narcotic combinations of
carfentanil-xylazine, etorphine-xylazine, carfentanil-xylazine-ketamine, or etorphine-xylazine-ketamine delivered
intramuscularly via dart to produce sternal recumbency. Oxygen was supplemented intra-nasally (3-5 L/min) in 25 of 35
immobilization events.
Samples were collected from the caudal auricular artery via a 20-ga needle into pre-heparinized
syringes. Analyses were done within 10 min of collection using an I-STAT portable blood gas analyzer (I-STAT Corporation,
Princeton, NJ 08540 USA), or an IRMA blood analysis system (Diametrics Medical Inc. St. Paul, MN 55113 USA) or stored on
ice for 3 hr and analyzed using CIBA-Corning 283 pH/ Blood Gas Analyzer (Bayer Diagnostics, Diagnostic Division
Tarrytown, NY 10591 USA). Parameters evaluated were pH, PaCO2, Pa O2,
HCO3 - and base excess (BE). Values were compared to acid base normals established for domestic cattle (Table
1).
Statistical Methods
The blood gas data were considered continuous and were evaluated for normality using the
Kolmogorov-Smirnov test with the null hypothesis of normality rejected at P < 0.05.
Analyses were performed to compare arterial blood gas data for manual, haloperidol, and immobilized
groups excluding animals with nasal oxygen supplementation. Within group comparisons for the immobilization group were
made at time 0 between animals supplemented and not supplemented with nasal oxygen. In addition, paired comparisons were
made between time 0 and time 30 for animals supplemented and not supplemented with oxygen. Multiple events on the same
animals were averaged.
Data that followed a normal distribution were compared between multiple groups using a one way, fixed
effect, analysis of variance. Where groups were different, multiple comparisons were performed using Tukey's test with
experiment-wise error set at alpha = 0.05. Data were compared between two groups using a t-test. Data that did not follow
a normal distribution were compared between multiple groups using the Kruskall-Wallis test. Where groups were different,
multiple comparisons were performed using Dunn's method with experiment-wise error set at alpha = 0.05. Data were
compared between two groups using the Wilcoxon Rank Sum Test. Paired comparisons were made using a paired t-test for
normal data and a Sign rank test for non-normal data.
All tests were performed against a two-sided hypothesis (SigmaStat v 5.0, SPSS Science, Tallahassee,
FL). A P < 0.05 was considered significant.
Results and Discussion
Results are reported in Table 2. Samples from all manually restrained bongo and eland indicated
metabolic acidosis (pH < 7.35 and HCO3-< 20). Hypocapnia (PaCO2 <
35) was observed in six of seven animals reflecting the expected compensatory response.
Haloperidol treated manually restrained animals (two bongo and one eland) maintained a normal pH
status and oxygenation but had a lower HCO3 (mean = 13.95) and BE (mean = -8.95) when compared to the manually
restrained acidotic animals. It is likely that haloperidol treated animals were able to compensate for the acid
production (most likely lactic acid) by lowering PaCO2 to < 35, whereas compensatory mechanisms
in manually restrained animals were overwhelmed.
Normal acid base values were observed in 42 % of the immobilization events. Forty-one percent of the
oxygen supplemented animals had a PaO2 # 92 mm Hg, whereas all unsupplemented animals were
hypoxemic. In 44 % of the events, animals had a-respiratory acidosis (pH < 7.35 and PaCO2 >
44) but showed little compensation (HCO3 > 30), presumably, because these disturbances were slight or
moderate. Two eland exhibited a respiratory alkalosis (pH > 7.45 and PaCO2 < 35). One was
euthanatized due to capture myopathy. One eland exhibited a metabolic alkalosis (pH > 7.45 and
HCO3-> 30). This animal died the day following immobilization and was found to have an enlarged,
flaccid heart. Histopathology was not performed. Arterial pH, PCO2, and HCO3 were much closer to
normal in the majority of the immobilized animals vs. the manually restrained animals that had a more severe metabolic
acidosis. Haloperidol, an anti-anxiety drug used in humans, may have reduced stress during manual restraint allowing
animals to maintain a normal acid base balance. Further evaluation of acid base status under various restraint condition
is encouraged. A flow chart to facilitate acid base interpretation is included (Fig. 1).
Table 1. Domestic cow reference values (George, 1994).
|
pH |
7.35-7.50 |
|
HCO3 - |
20-30 |
|
PCO2 |
35-44 |
|
PO2 |
92 |
Table 2. Blood gas measurements in bongo and eland using three different restraint methods.
|
Parameters
measured |
Manual restraint |
Haloperidol |
Immobilized |
|
Arterial |
Arterial |
Arterial - O2
supplemented |
Arterial - no O2
supplemented |
|
|
n = 6 |
n = 3 |
n = 25 |
n = 7 |
|
pH |
|
mean |
7.19 |
7.36 |
7.36 |
7.38 |
|
median |
7.23 |
7.36 |
7.34 |
7.39 |
|
range |
7.01-7.30 |
7.35-7.38 |
7.23-7.53 |
7.32-7.47 |
|
PaCO2 |
|
mean |
26.15 |
24.60 |
46.80 |
44.27 |
|
median |
26.35 |
24.60 |
45.00 |
45.40 |
|
range |
22.70-29.20 |
24.60-24.60 |
24.00-67.00 |
34.10-48.00 |
|
PaO2 |
|
mean |
112.85 |
110.10 |
106.19 |
70.57 |
|
median |
114.65 |
122.60 |
101.00 |
68.00 |
|
range |
98.80-123.30 |
81.60-126.10 |
16.00-214.00 |
57.00-86.00 |
|
HCO3- |
|
mean |
10.00 |
13.95 |
25.41 |
26.34 |
|
median |
10.60 |
13.95 |
25.70 |
26.00 |
|
range |
8.20-14.20 |
13.50-14.40 |
13.00-32.00 |
21.00-32.00 |
|
BE |
|
mean |
-14.33 |
-8.95 |
0.24 |
1.30 |
|
median |
-14.60 |
-8.95 |
-0.67 |
0 |
|
range |
-18.60--9.50 |
-9.60--8.30 |
-4.60-5.0 |
-4.00-8.00 |
| Figure 1. Acid base interpretation flow chart.2 | 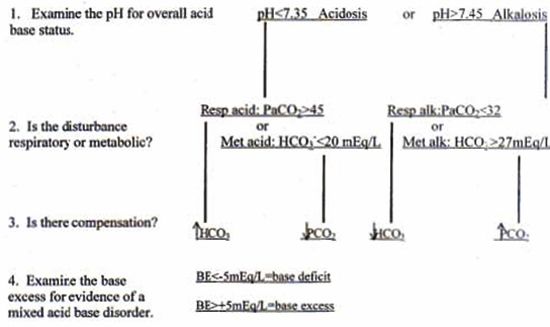
|
|
| |
References
1. George JW. 1994. Water electrolytes and acid base. In: Duncan, R. J., K. W. Prasse, and
E. A. Mahaffey (eds.). Veterinary Laboratory Medicine Clinical Pathology. 3rd ed., Ames IA, Iowa State University
Press, Pp 94-111.
2. Wingfield WE. 1997. Acid-base disorders. In: Veterinary Emergency Secrets. Hanley
and Belfus, Inc. Philadelphia, PA. Pp. 288-293.