Abstract
Background
An epizootic involving a neutrophilic leukocytosis, increased erythrocyte sedimentation rate, and inappetance affected approximately 70% of the U.S. Navy Marine Mammal Program's (NMMP) Tursiops truncatus (Atlantic bottlenose dolphin) population during July-October 2004. While a viral etiology was suspected, the only virus isolated proximate to the time of the epizootic was an enterovirus from a tongue lesion in a 16-year old female dolphin, 'AND', during June 2004.
To assess the potential role of the AND enterovirus during the epizootic, the Oklahoma Animal Disease Diagnostic Laboratory (OADDL) developed a serum neutralization assay. Seroprevalence studies were conducted with the Navy's dolphin population in San Diego, California, and due to their potential role as sentinels for enterovirus exposure, wild sea lions at The Marine Mammal Center (TMMC) in Sausalito, California were also screened. A cohort study was conducted comparing complete blood counts, serum chemistries, and clinical signs among dolphins that had evidence of active infection (at least a four-fold change in antibody titer) during the epizootic and those that did not have evidence of active infection.
Isolation and Characterization of Enterovirus
Each submitted oral swab in approximately 5 ml of M4 medium was filtered through a 0.22µm filter and the filtrate was used to inoculate one-day-old cultures of Crandle-Reese Feline Kidney (CRFK) and Vero (African green monkey kidney) cell lines in a 24-well plate. After a 45-min incubation, the cell monolayers were overlaid with 1 ml per well of Dulbecco's modified Eagle's Medium (Cellgrow, Mediatech, Inc. supplemented with 5% fetal bovine serum, 2mM L-glutamine, 200U/ml/200 mg/ml Penicillin/Streptomycin, 10mM Hepes buffer, (DMEM complete media + 5% FBS)). Inoculated cells were incubated in a CO2 incubator at 37°C. No cytopathic change was observed after 6 days.
Cell cultures were passaged by trypsinization (Trypsin-EDTA (Cellgrow, Mediatech, Inc.). After 5 days in the second passage, cytopathic changes were observed in the Vero cell line. A chloroform test indicated the virus isolated was nonenveloped. PCR confirmed the virus to be an enterovirus and sequencing of PCR products indicated the virus to be indistinguishable from bovine enterovirus type 1 (BEV-1).
Development of the Serum Neutralization Assay
The serum neutralization test (SNT) was performed using the AND enterovirus. The virus was grown in Vero (African green monkey kidney) cells and the test was performed following a modification of a microtiter method (Rossiter et al., 1985) as previously described for morbilliviruses (Saliki et al., 1993). Briefly, serial twofold dilutions of heat-inactivated sera were made in duplicate columns of 96-well plates using the DMEM complete media starting at a 1:2 dilution. An equal volume (25 µl) of virus containing about 100 TCID50 was added. Addition of virus yielded a starting dilution of 1:4. The virus-serum mixtures were incubated at 37°C for 1 hour in 5% CO2 and a Vero cell suspension (150 µl containing 104 cells/well) was added to the plates. The plates were incubated at 37°C in 5% CO2 for 3 days. The test was read by examining cell monolayers under an inverted microscope for virus-specific cytopathic effects (CPE). Antibody titers were expressed as the reciprocal of the highest dilution of serum that completely neutralized CPE in duplicate wells.
Seroprevalence Studies
Tursiops truncatus population at the NMMP. A total of 200 serum samples, collected during 1998-2004 from 53 dolphins, were tested for antibodies to the AND enterovirus. At least three samples were submitted from each dolphin, including one sample collected approximately 1 year previous to the epizootic, one sample collected at the onset of a neutrophilic leukocytosis (absolute neutrophil count > 7,543/ul and WBC count > 10,200/ul) between July-October 2004, and one sample collected four weeks after the initial onset of the neutrophilic leukocytosis. Of the serum samples analyzed, 95 (48%) were from females, and 105 (52%) were from males; the median age of animals at the time of sample collection was 22.2 years (range 0.6-47.2 years). A total of 168 (84%) serum samples from 51 (96%) dolphins were analyzable (clean); of these, 167 (99%) samples from 51 (100%) dolphins had detectable antibodies to the AND enterovirus. Titer ratios of positive animals varied from 1:4 to > 1:256; the median titer ratio was 1:24 (Graph 1).
Zalophus californianus population at TMMC. A total of 57 paired serum samples, collected during January to December 2004 from 27 sea lions, were tested for antibodies to the AND enterovirus. At least two samples were submitted from each sea lion, including one sample collected at the onset of a clinical presentation to TMMC and one sample collected approximately four weeks after initial presentation. A total of 53 (93%) serum samples from 27 (100%) sea lions were analyzable (clean); of these, 53 (100%) samples from 27 (100%) sea lions had detectable antibodies to the AND enterovirus.
Titer ratios of positive animals varied from 1:4 to > 1:256; the median titer ratio was 1:192 (Graph 1).
When comparing the two marine mammal populations, sea lions were significantly more likely to have higher antibody titer ratios than dolphins (p-value < .0001). While it appears that levels of enterovirus antibodies may be higher in sea lions compared to dolphins, the difference due to location (northern v. southern California) could not be ruled out as a confounder. Follow-up seroprevalence studies are being conducted on the NMMP sea lion population to address this issue.
| Graph 1. | 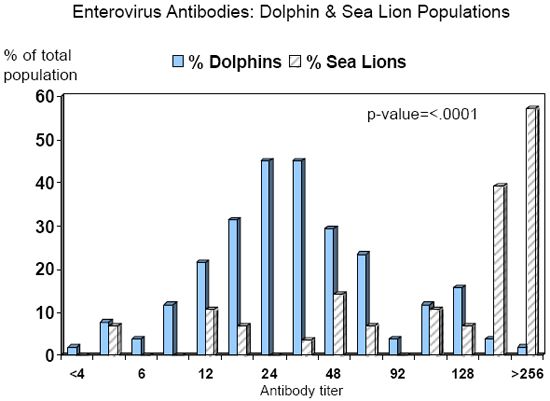
Seroprevalence of enterovirus antibodies in two marine mammal populations, years 1998-2004. |
|
| |
Dolphin population = U.S. Navy Marine Mammal Program in San Diego,
California Sea lion population = The Marine Mammal Center in Sausalito, California
Additional Species. Upon development of the serum neutralization assay, OADDL tested 12 additional animal groups for antibodies to the AND enterovirus (Table 1). Results demonstrated that marine mammals (except the sea otter) and bovine were more likely to have detectable antibodies to the AND enterovirus compared to non-bovine terrestrial species. These results support an established marine-based virus of potential terrestrial origin.
Table 1. AND Enterovirus titer ratios in various animal groups.
|
Animal group |
AND enterovirus
titer ratio |
|
Sperm whale |
1:24 |
|
Walrus |
1:64 |
|
Seal |
1:32 |
|
Bovine |
1:24 |
|
Canine |
1:8 |
|
Equine |
1:4 |
|
Caprine |
<1:4 |
|
Feline |
<1:4 |
|
Llama |
<1:4 |
|
Ovine |
<1:4 |
|
Porcine |
<1:4 |
|
Sea Otter |
<1:4 |
Cohort Study: CBCs, Serum Chemistries, & Clinical Signs
CBCs and Serum Chemistries. A total of 41 dolphins were identified as epizootic cases during July 2004, where cases were defined as an animal with a neutrophilic leukocystosis and at least a two-fold increase in erythrocyte sedimentation rate. To assess the potential role of enterovirus during the epizootic, a cohort study was conducted to compare dolphins with evidence of active enterovirus infection with those without evidence of active infection. Of the 41 epizootic case animals, 15 (37%) had at least a four-fold change in enterovirus antibody titers over a four-week period during the epizootic; 26 (63%) had less than a four-fold change in titer. Animals with a four-fold change in enterovirus titers during the epizootic were more likely to have higher absolute lymphocyte counts, liver transaminases, creatine kinase, and erythrocyte sedimentation rate; and lower hematocrit compared to animals without a four-fold change in enterovirus titers (Table 2). There were no significant differences in total white blood cell counts or absolute neutrophil counts when comparing the two groups (p-value=0.31 and 0.13, respectively).
Table 2. Comparison of mean blood values by active / non-active enterovirus infections, Tursiops truncatus, July-October 2004.
|
Blood Variable |
Mean Value,
4-fold change in
enterovirus titer |
Mean Value,
No 4-fold change in
enterovirus titer |
p-value |
|
WBC |
19,399 |
20,867 |
0.31 |
|
Neutrophils |
15,461 |
17,936 |
0.13 |
|
Lymphocytes |
1,600 |
1,152 |
0.02 |
|
HCT |
38.2 |
40.8 |
0.01 |
|
LDH |
496.3 |
360.2 |
0.0003 |
|
AST |
413.9 |
188.7 |
< .0001 |
|
ALT |
92.8 |
37.7 |
0.0008 |
|
CPK |
105.2 |
61.0 |
< .0001 |
|
ESR |
82.7 |
50.2 |
0.02 |
Clinical Signs. Clinical signs reported during the epizootic included inappetance, vomiting, diarrhea, body cavity effusions, and squinting +/- corneal opacities. Of these clinical signs, only squinting +/- corneal opacities were significantly associated with active enterovirus infection during the epizootic (8/15, 53.3% v. 4/26, 15.4%; p-value =0.01).
While there is evidence that active enterovirus infections caused an increase in lymphocyte counts, liver transaminases, and ophthalmic clinical signs in the NMMP dolphin population during July-October 2004, the cohort study results do not support the hypothesis that enterovirus was the cause of the 2004 epizootic.
Summary
The seroprevalence and cohort studies described above support that enterovirus is an established virus in the marine environment, including marine mammals, and may have originated from bovine species. Sea lions may have higher antibody levels than bottlenose dolphins, but follow-up studies comparing sea lion populations in both northern and southern California are needed. There is evidence that active enterovirus infection is associated with increased lymphocyte counts, liver transaminases, and erythrocyte sedimentation rate; additionally, active infection was associated with ophthalmic clinical signs in bottlenose dolphins, including squinting / photophobia +/ -corneal lesions. Additional studies to further characterize the morbidity of enterovirus in marine mammal populations are warranted.
Acknowledgments
The authors thank the staff at the Navy Marine Mammal Program, The Oklahoma Animal Disease Diagnostic Laboratory, and The Marine Mammal Center for their support with this project.
References
1. Rossiter PB, DM Jessett, WP Taylor. 1985. Microneutralisation systems fo r use with different strains of peste des petits ruminants virus and rinderpest virus. Trop. Anim. Hlth. Prod. 17:75-81.
2. Saliki JT, G Libeau, JA House, CA Mebus, EJ Dubovi. 1993. Monoclonal antibody-based enzyme-linked immunosorbent assay for specific detection and titration of peste-des-petits -ruminants virus antibody in caprine and ovine sera. J. Clin. Microbiol. 31:1075-1082.
3. Smith AW, DE Skilling. 1979. Viruses and virus diseases of marine mammals. J. Am Vet Med Assoc. 175(9):918-920.
4. Wong SK, CR Smith, W Van Bonn, ED Jensen, SR Ridgway. 2005. Investigation of an Epizootic of Inappetance in a Tursiops truncatus population. IAAAM Proceedings.