Jill E. Maddison, BVSc, PhD, FACVSc, MRCVS
There has been a major shift in the recognition and management of pain in animals over the past 20 years. In the past, human and veterinary physicians were trained to emphasise objective data only and to consider only the observed and measurable aspects of a patient's response to noxious stimuli. Ignoring pain in animals has been historically socially well established as animals were largely valued in economic terms and veterinary medicine was primarily concerned with restoring productivity if possible. Keeping animals free of pain was not a concern and even today, attitudes to pain management in large and small animal practice differ markedly. The use of postoperative analgesia in small animals has only been a widespread practice in the 1990's.
OPIOID ANALGESICS
Morphine, pethidine, methadone, codeine, fentanyl, buprenorphine, butorphanol
The opioid analgesics remain the most potent analgesic drugs in veterinary medicine. The standard drug is morphine, named after the Greek god of dreams, Morpheus. Although these drugs can have significant side effects, for most animals in serious pain, these are usually not of sufficient concern to prevent use of these drugs to relieve pain.
Opioids are most effective for treatment of moderate to severe pain particularly acute pain due to trauma and surgical procedures. The major side effects that may be of concern in critically ill patients are respiratory depression, hypotension and bradycardia.
The efficacy of the opioids varies. Pure µ receptor agonists such as morphine, oxymorphone, methadone and fentanyl provide better pain relief than partial agonists or agonist-antagonists such as buprenorphine, pethidine (meperidine) and butorphanol. Thus the pure agonists are best for orthopaedic procedures which are particularly painful primarily as a result of pain receptors in the periosteum.
There are several studies that suggest that newer nonsteroidal anti-inflammatory drugs such as carprofen, ketoprofen, meloxicam and etodolac are equally or more efficacious analgesically and are longer acting than the synthetic opioids especially pethidine and butorphanol. It should be noted however that these opioids have less efficacy than the pure agonists such as morphine and methadone.
The duration of action of opioids also varies significantly (see table).
Side effects (using morphine as the prototype)
Although dose related respiratory depression is the major side effect of morphine, most postoperative patients can tolerate the mild to moderate depression that occurs with therapeutic doses. In addition, the respiratory depressive effects of the opioids are at least partially counteracted by the respiratory stimulatory effects of pain. Clinically significant hypoventilation is rarely a problem unless high doses (> 1 mg/kg) are used. However, animals with head injury are at particular risk from the respiratory depressant effects because hypercapnia increases cerebral blood flow.
Morphine administration causes vasodilation which promotes pooling of blood in peripheral veins and the liver. This, in combination with respiratory depression can be beneficial to the patient with pulmonary oedema, particularly if the oedema is due to cardiac failure.
Other side effects associated with the administration of morphine include histamine release, nausea and vomiting and dysphoria (disquieted state accompanied by restlessness and a feeling of malaise) particularly when administered intravenously too quickly
Opioid Dosages and Duration of Action
|
Drug |
Route of Administration |
Dose Rate (mg/kg)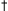 |
|
Duration of
Analgesic Action (hr)# |
|
|
|
Dog |
Cat * |
|
|
Morphine |
IV |
0.05-0.1 |
0.05 |
1-4 § |
|
|
Intraoperative bolus |
0.1 |
0.05 |
|
|
|
IM or SC |
0.1-1.0 |
0.1-0.3 |
4-6 |
|
|
Epidural |
0.1 |
0.1 |
12-24 |
|
|
Interpleural |
0.5-1.0 |
|
8-12 |
|
|
Intra-articular |
0.1-1.0 |
|
8-12 |
|
|
Oral |
0.1-3.0 |
0.1-1.0 |
4-8 |
|
|
Sustained release oral |
1.5-3.0 |
|
8-12 |
|
Pethidine |
IV |
Contraindicated in small animals |
|
|
IM or SC |
2-10 |
2-10 |
1-2 |
|
Methadone |
Iv |
0.05-0.01 |
0.05-0.01 |
4-6 § |
|
|
IM or SC |
0.1-1.0 |
0.1-1.0 |
4-6 |
|
Fentanyl |
IV intraoperative bolus |
0.002-0.005 |
0.001-0.005 |
0.3-0.5 |
|
|
Transdermal |
|
|
|
|
|
Patch sizes: 25, 50, 75 & 100 µg/hr |
2-4 µg/kg/hr |
2-4 µg/kg/hr |
Up to 72 |
|
|
With Droperidol-(Leptan / Innovar-Vet) |
|
|
|
|
|
IV induction |
0.04 ml/kg |
Contraindicated |
|
|
|
IM or SC |
0.02-0.04 ml/kg |
|
0.5-1.0 |
|
Alfentanil |
IV |
0.01-0.025 |
|
|
|
Buprenorphine |
IV |
0.005-0.02 |
0.005-0.01 |
3-4 |
|
|
|
0.005-0.02 |
0.005-0.01 |
4-12 |
|
Butorphanol |
IV |
0.2-0.4 |
0.2-0.4 |
1-3 (d), 4 (c) |
|
|
IM or SC--analgesia |
0.2-0.4 |
0.2-0.4 |
2-6 |
|
|
IM or SC--antitussive |
0.05-0.1 |
|
6-12 |
|
|
Oral |
0.2-1.0 |
0.2-1.0 |
6-8 |
 Dose selected will depend on desired effect, whether for sedation and/or analgesia, and other drugs concurrently administered.
Dose selected will depend on desired effect, whether for sedation and/or analgesia, and other drugs concurrently administered.
* Where no dose rate is given for cats there are generally no recommendations available in the literature rather than that drug being contraindicated for use in cats. A similar or lower dose may be used with caution.
# Duration of action will be affected by concurrent drug administration, desired effect and type of procedure performed and therefore act as a guide only. These variables will influence the repeat dosing schedule and hence each animal should be assessed for evidence of pain and response to therapy.
§ IV boluses can be "titrated" to effect by administering a bolus from the lower end of the dose range and repeating every 5-10 minutes until the desired effect is achieved. At the same time it is important not to overdose the patient.
Adapted from Nicholson, AN and Christie M (2001) Opioid Drugs in Small Animal Pharmacology eds. Maddison, JE, Page SW and Church DB. Saunders.
Histamine release causes systemic hypo-tension which can worsen circulatory shock. However, appropriate fluid therapy usually prevents significant problems. Bradycardia occurs with all opioids except pethidine and is commonly observed in anaesthetized animals. Serious bradycardia is uncommon and can usually be managed by treatment with atropine.
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
The use of non-narcotic analgesics, specifically non-steroidal anti-inflammatories (NSAIDs) in the management of pain associated with acute trauma or surgery is receiving increasing attention in small animal practice. NSAIDs do not produce sedation or ataxia and allow more rapid recovery from anaesthesia (if this is desired). They are also particularly effective analgesics when the cause of pain is inflammation.
Mechanism of action
Nonsteroidal anti-inflammatory drugs (NSAIDs) have anti-inflammatory, antipyretic and analgesic activity and inhibit platelet aggregation. Their mechanism of action includes inhibition of the production of several mediators of inflammation, including prostaglandins and thromboxane via inhibition of the enzyme cyclo-oxygenase. The other major products of arachidonic acid metabolism are the leukotriene series, the production of which is mediated by the enzyme lipoxygenase.
Prostaglandins in particular PGE2 sensitises receptors on afferent nerve endings to agents that cause pain e.g., bradykinin, serotonin and histamine. PGE2 is also, a potent pyretic agent, stimulated by IL-1 in bacterial and viral infections and a potent dilator of vascular smooth muscle.
COX-1, COX-2 and COX-3
There are now believed to be three forms of cyclo-oxygenase, COX-1, COX-2 and COX-3. It is thought that COX-1 is responsible for the production of prostaglandins important in the physiological modulation of function (e.g., prostacyclin), whereas induction of COX-2 is associated with production of inflammatory prostaglandins and hence disease processes. The discovery of COX-2 in the 1980s revolutionised NSAID pharmacology by providing a clear mechanism for prostaglandin regulation in vivo and by providing a new target for the development of COX-2 selective drugs. The search for an additional COX isoenzyme was stimulated by the observation that paracetamol (acetaminophen) was an effective analgesic and antipyretic but was not anti-inflammatory. In 1972 Flower and Vane using canine brains as their enzyme source postulated that paracetamol acted centrally by inhibiting COX enzymes. However, numerous studies showed that COX-1 and particularly COX-2 are relatively insensitive to paracetamol. The identification of COX-3 partially solved this dilemma as it is significantly more sensitive to the effects of paracetamol than COX-1 and COX-2 (Simmons, 2003).
COX-1 is involved in the production of prostaglandins important in the physiological modulation of function especially:
 gut mucosal barrier
gut mucosal barrier
 intra-renal perfusion when renal blood flow is decreased
intra-renal perfusion when renal blood flow is decreased
COX-2 is activated and released by:
 tissue damage
tissue damage
 bacterial lipopolysaccharide
bacterial lipopolysaccharide
 cytokines
cytokines
 growth factors
growth factors
 inflammation where PGE2 is predominant eicosanoid
inflammation where PGE2 is predominant eicosanoid
COX-3 appears to be involved with central pain relief at least in dogs. It is possible that it may not be as important in humans.
The ratio of COX-1:COX-2 effect varies with different drugs and in part explains why some NSAIDs are more ulcerogenic than others. However, the picture is far from clear as results published in relation to the relative COX-2 preference of a drug will be influenced by the assay chosen (whole blood assay is the gold standard), the tissue type, the species used and the relative degree of inhibition of COX-1 that occurs at pharmacologically and clinically relevant levels of COX-2 inhibition (<80%).
Although preferential COX-2 inhibition appears to be an important factor in improving the safety of NSAID drugs, this is not the only consideration. Other factors that are involved in producing safer NSAIDs involve reducing the acidity of the pro-drug, shortening plasma half life and reducing enterohepatic recycling.
COX-2 may also have important physiological functions. For example, COX-2 is expressed constitutively in the kidney, particularly by the macular densa and has a role in the control of renin release. It has also been demonstrated that COX-1 expression may play a role in inflammation and therefore agents that are selective COX-2 inhibitors may not be as efficacious as anti-inflammatory agents as drugs which inhibit both COX-1 and COX-2.
The duration of effect of NSAIDs exceeds their biological half-life. This is thought to be due to the concentration of NSAIDs in locations where the pH of extracellular fluid is decreased such as at sites of inflammation.
Side effects
The ability of NSAIDs to reduce the production of prostaglandins and thromboxane and thus reduce inflammation, is also responsible for the potential toxicity of this drug class.
Prostaglandins play an important role in a wide variety of body functions. Of most relevance to NSAID toxicity is the role of PGI2 (prostacyclin) and PGE2 in maintaining the integrity of the protective barrier that prevents gastric mucosa from damage by gastric acid.
Gastric ulceration
All NSAIDs have the potential to cause gastric ulceration by inhibiting the production of PGE and PGI2. One of the problems in canine medicine in particular, is that dogs appear more sensitive to the gastrointestinal side effects of NSAIDs than other species, possibly as a result of increased enterohepatic recycling of the drugs in this species and therefore longer half lives of the drugs.
The ulcerogenic potential of NSAIDs is increased by: concurrent corticosteroids, dehydration, hypovolaemic shock, disruption to normal gut blood flow.
Renal toxicity
A second potential side effect of NSAIDs is renal toxicity as a result of reduced renal blood flow and glomerular filtration rate secondary to inhibition of renal prostaglandin synthesis. Renal prostaglandins are involved in maintaining renal blood flow via their vasodilatory actions. In a healthy, well hydrated animal reduced renal prostaglandin production is of little consequence. However, significant renal toxicity can result if an animal is volume depleted, is avidly retaining sodium (e.g., in congestive heart failure or hepatic cirrhosis) or has pre-existing renal insufficiency.
The relative risk of renal toxicity in a volume depleted dog is not reduced by using a COX-2 preferential drug as both COX-1 and COX-2 have important physiological functions in the kidney.
Haematology and coagulation
Prolongation of bleeding times due to inhibition of platelet thromboxane production can occur after administration of any of the NSAIDs. However, it is potentially more serious with use of those drugs which irreversibly bind to cyclo-oxygenase such as aspirin and phenylbutazone, as the effect persists for the life of the platelet (which is unable to synthesis additional thromboxane as it lacks a nucleus).
References
1. Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol). Nature (1972) 240:410-411.
2. Simmons DL Variants of cyclooxygenase-1 and their roles in medicine. Thrombosis Research (2003) 110:265-268