Veterinary Clinical Sciences School of Veterinary Medicine, Louisiana State University
Baton Rouge, LA, USA
Nosocomial (NI) or hospital acquired infections (HAI) have been recognized as a problem since the 19th century in human medicine. In 1846 at the General Hospital of Vienna Dr. Ignaz Semmelweis observed that women whose babies were delivered by students and physicians who had previously performed autopsies consistently had a higher mortality rate than those whose babies were delivered by midwives. He implemented a disinfection program using chlorinated lime solution which significantly reduced mortality. More than 150 years later (2002), HAI have become the 6th most common cause of death in the USA with almost 100,000 fatal cases and an estimated 1.7 million cases of HAI registered in US hospitals. Similar numbers have been reported in many other countries worldwide. HAI are defined as infections that develop more than 48 hrs after admission to the hospital. In the US, ¼ of the cases happened in intensive care units (ICU) while ¾ occurred outside of ICUs. Based on these numbers, the additional burden on the health care system is estimated to be in the range of billions of dollars. The main issues associated with NI in human hospitals revolve around transmission of multi-drug resistant (MDR) micro-organisms from patient to patient by health care personal and management of patients infected with micro-organisms resistant to all antibiotics such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus and others.
An increasing number of reports of HAI and MDR bacteria is available in the small animal veterinary literature. These publications clearly show that NI are also a constant threat for dogs and cats in referral centers as well as in general practice. However, their incidence remains smaller than what is observed in the human health care system. This may be due to factors such as shorter hospital stays (especially in the ICU), lower prevalence of immunosuppressive diseases, and absence of long-term care facilities in small animal medicine. The main issues associated with NI in dogs and cats are transmission of MDR micro-organisms from patient to patient by veterinary staff (e.g., in ICU), animals with infectious diseases contagious to other animals (e.g., distemper, parvovirosis, feline calici- and herpes viruses), and zoonoses that could affect veterinary staff and possibly the general public (e.g., MRSA). However, the situation is changing rapidly: the improvement of standards of care for critical small animal patients is one factor that may lead to an increase in NI. MDR bacteria could become a serious threat in our practices and hospitals very soon if we do not act at this time.
Etiology/Pathogenesis
Numerous infectious agents such as viruses, bacteria, fungi, and protozoan parasites can spread as nosocomial infections in a small animal hospital. Greene (2006) divides these agents in 4 classes according to the ease of their transmission and their zoonotic potential. Dogs and cats with class 4 diseases (transmission by infected body secretions with organisms of moderate environmental resistance and zoonotic potential) must be kept isolated from other hospital patients (e.g., parvovirosis, feline upper respiratory tract diseases). Animals with class 3 diseases (zoonotic potential with direct transmission to people) should be carefully handled during their hospital stay (e.g., leptospirosis, campylobacteriosis, giardiasis).
In the absence of such infectious diseases, NI arise from hospital acquired microorganisms or bacteria from the patient's normal intestinal or skin flora. Figure 1 illustrates the fact that the occurrence of NI is preceded by colonization of mucosal surfaces by these bacteria. Patients weakened by their primary disease (an intrinsic factor) and undergoing invasive procedures often also receive multiple antibiotics due to their primary disease condition (extrinsic factors). This makes them very susceptible to the development of NI.
| Figure 1. Development of nosocomial infection. | 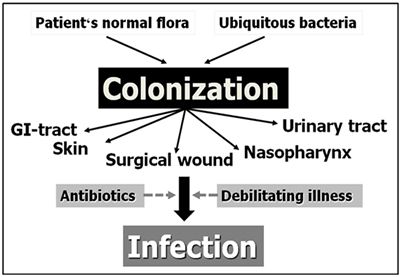
|
|
| |
Invasive procedures routinely performed in small animal hospitals include placement of indwelling urinary catheters, central venous lines, chest drains, and of course surgical interventions. Biofilm consists of a layer of microorganisms that produce a matrix allowing them to adhere on the surface of most indwelling or medical external devices and colonize the anatomical area. Biofilm is an essential step in the pathogenesis of catheter-induced NI.
Recently, a number of studies identified the risk factors for surgical site infections: surgical site clipping and aseptic preparation before surgery, duration of surgery, duration of anesthesia, emergency vs. daytime surgery, surgical tissue handling, number of persons present in the operating room, total length of hospital stay, etc.
Commonly isolated MDR bacteria in small animal veterinary facilities include E. coli, Enterobacter, Enterococcus spp., as well as various Staphylococcus spp. and Clostridium spp. In Europe, Acinetobacter baumannii was identified as a resistant bacteria causing NI in the small animal and equine units of a veterinary teaching hospital.
Approach of Declared NI
In small animal veterinary practices and hospitals the most common type of NI are urinary tract infections, followed by surgical site infections and blood stream infections. When such infections with MDR bacteria are diagnosed in hospitalized patients, it is important to record the circumstances under which they appeared. Which invasive procedures were performed? How were catheters, drains and wounds taken care of during the stay in the ICU? In which area of the hospital was the animal kept? This information is essential to detect what mistakes were made, to identify them and implement a hospital policy to improve these points.
In order to prevent spread of MDR bacteria to other patients, veterinary staff treating animals with documented MDR infections should be cautious and take all necessary measures to contain the infection. The single most efficient method to limit the spread of NI is hand washing with soap for at least 1 minute if the hands are visibly dirty, or hand rubbing with a 70% alcohol solution or foam for 20-30 seconds if the hands appear clean, before and after treating each patient. Use of latex gloves may be helpful in order to limit exposure of veterinary staff to the MDR bacteria--however, this is only useful if the hands are rubbed with alcohol immediately after the gloves are removed. Latex gloves only prevent the spread of MDR bacteria within the veterinary facility if they are changed after each contact with a patient, and this is often not practical. Finally, additional barrier precautions must be considered based on the conditions present in the hospital.
Prevention of NI
The most important aspect of the approach of NI is clearly their prevention. Several simple strategies can effectively reduce the risk of NI in small animal patients. First, limitation of the extrinsic risk factors is the most logical approach. Invasive procedures should only be performed if absolutely necessary. Surgical interventions should be designed to avoid the many risk factors for surgical site infections described above. Indwelling urinary catheters should be removed as soon as the patient's condition allows it. The consequences of antibiotic use in dogs and cats with indwelling urinary catheters should also be evaluated.
Proper care of intravenous and urinary catheters is essential to prevent complications. It begins with respecting the appropriate protocol for asepsis prior to and during placement of the catheter. Proper device fixation should be in place. Finally, manipulations of the catheters must be preceded by hand washing with soap or rubbing with alcohol-based solution of foam (see above).
The prevalence of antimicrobial resistance in nosocomial microorganisms affecting small animals is increasing rapidly. The only way to influence this development is to restrict the use of antibiotics to animals proven to suffer from bacterial infections, and to design and enforce a clear antimicrobial use policy. Such policies should be tailored to each veterinary practice or hospital, and aim at defining which drugs are used as 1st line antibiotics. Selection and use of antibiotics should be guided by results of culture and sensitivity of isolated bacteria. If tissue sampling for culture is impossible or impractical, the selected antibiotics should be directed against the type of bacteria most likely to cause the disease, in consideration of their usual antimicrobial susceptibility and of the achievable drug levels at the site of infection. Indiscriminate use of the newest broad spectrum antibiotics ('big guns') is a sign of short-sightedness on the side of the veterinarian, as this will invariably increase the risk of bacterial resistance developing rapidly against these agents in his/her immediate environment. Use of 'last resort drugs' from human medicine such as imipenem or vancomycin in dogs and cats is associated with a risk of spreading resistance to these agents, and is therefore a concern for public health.
Finally, a surveillance program should be established in small animal practices and hospitals where NI were documented. Such a program may include monitoring of all bacteria isolated from dogs and cats in the hospital with special attention to the occurrence of MDR microorganisms. Implementation of efficient prevention measures must be accompanied by regular information of all veterinary staff involved in the care of animals in the facility. It is a proven fact that well informed caregivers are more likely to follow the basic principles necessary to prevent the occurrence of NI.
Conclusions
Nosocomial infections and multi-drug resistant bacteria are challenges small animal veterinarians must face at this time. These problems cannot be ignored any longer, as it will ultimately influence the success and the quality of care we can deliver to our canine and feline patients. Implementing basic preventative measures as delineated above and monitoring the results of bacterial cultures are useful first steps in this endeavor.
References
1. Boerlin P, Eugster S, Gaschen F, et al (2001). Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol. 82:347-59.
2. Bubenik LJ, Hosgood GL, Waldron DR, et al (2007). Frequency of urinary tract infection in catheterized dogs and comparison of bacterial culture and susceptibility testing results for catheterized and non-catheterized dogs with urinary tract infections. J Am Vet Med Assoc. 231(6):893-9.
3. Eugster S, Schawalder P, Gaschen F, et al (2004). A prospective study of postoperative surgical site infections in dogs and cats. Vet Surg. 33:542-50.
4. Francey T, Gaschen F, Nicolet J, et al (2000). The role of Acinetobacter baumannii as a nosocomial pathogen for dogs and cats in an intensive care unit. J Vet Intern Med. 14:177-83.
5. Greene CE (2006): Nosocomial Infections in Greene CE (ed): Infectious diseases of the dog and cat. 3rd edition. Saunders Elsevier, St. Louis, MO; 997-1007
6. Ogeer-Gyles J, Mathews K, Weese JS, et al (2006). Evaluation of catheter-associated urinary tract infections and multi-drug-resistant Escherichia coli isolates from the urine of dogs with indwelling urinary catheters. J Am Vet Med Assoc. 229:1584-90.
7. Ogeer-Gyles JS, Mathews KA, Boerlin P (2006): Nosocomial infections and antimicrobial resistance in critical care medicine. J Vet Emerg Crit Care 16: 1-18.