Humoral Immune Response to DNA-Mediated Vaccination in African Black-Footed Penguins (Spheniscus demersus) and Chain Dogfish (Scyliorhinus retifer)
Abstract
Immunization using DNA, or nucleic acid vaccination, represents a potentially powerful, efficacious, and low-risk method for vaccination of
valuable aquatic species kept in aquaria and zoos (e.g., teleosts, elasmobranchs, penguins, pinnipeds, and cetaceans) against common species-specific diseases
associated with high mortality or morbidity. Factors contributing to successful plasmid vaccination of penguins and elasmobranchs have not been reported to date.
This study investigates humoral responses of African black-looted penguins (Spheniscus demersus) and chain dogfish (Scyliorhinus retifer) inoculated
with a Beta-galactosidase reporter gene expression plasmid.
Plasmids are short, circular pieces of DNA that can be engineered to contain nucleic acid sequences encoding an antigen of interest known to
elicit a protective immune response in the host species. Plasmid vaccines stimulate specific responses from all arms of the immune system which is vital to
development of protective immunity in the host.3,5,9,12,14-16 Nucleic acid vaccination induces long-lasting humoral, cellular, and mucosal immunity
because protein antigens are directly expressed in their native architecture by host cells. This process insures the induction of conformation-dependent
antibodies, which are important first-line products of an immune response. While the most common route of administration of plasmid DNA is by intramuscular (IM)
injection1,2,7,10,11,13, intradermal (ID) injection and mucosal absorption have also been successful.4,6,8
This study employs the use of a reporter gene plasmid containing the gene for l galactosidase, an enzyme that bacteria (Escherichia
coli) use to split lactose into simple components, glucose and galactose. A reporter gene plasmid contains a gene whose protein product is foreign to the host
but is not pathogenic. The protein the gene encodes can be easily assayed for, and thus it "reports" its own expression in the host cell and allows measurement of
an immune response. When bacterial Beta-galactosidase is expressed in mammalian cells, it is considered a foreign protein and an immune response is mounted
against it. However, the protein itself is not harmful to the host animal. Any antigen made is broken down by host cell enzymes and does not accumulate. In this
manner, the reporter gene system allows examination of the test animal's immune response to a foreign protein without the use of material from pathogenic
organisms.
The humoral response to plasmid vaccination was investigated in twelve adult penguins. On Day O, six birds received 100 microliters of sterile
phosphate-buffered saline (PBS) by IM injection, three received 50 micrograms of the control plasmid pCI in PBS (lacking the gene for Beta-galactosidase), and 3
received 50 micrograms of the test plasmid pCMV-Beta in PBS (containing the gene for Beta-galactosidase). Blood samples were drawn weekly to harvest serum for
antibody screening. After three weeks, the six birds that received saline were divided equally, assigned to the other two groups, and given the appropriate
injection of test or control plasmid. This approach was used to achieve group sizes of six birds for each of the three conditions, while minimizing the total
number of animals (12) devoted to the study. Blood samples were obtained weekly for six weeks post-inoculation from all birds. Although the six birds initially
receiving saline were out of phase secondarily, they were monitored for 42 days post-inoculation with plasmid in the same manner as the others within the
corresponding pCI or pCMV-Beta plasmid groups. A "booster" vaccine (identical to the first vaccine) was given to the twelve plasmid-vaccinated birds on Day 21.
Penguin sera were screened for antibodies against Beta-galactosidase using ELISA immunoassays. Anti-chicken IgG was shown to have an
acceptable binding efficacy to penguin serum immunoglobulins and thus was employed as the secondary antibody for the ELISAs. Each sample from each bird was run in
duplicate on 96-well plates at serial doubling dilutions starting at 1:20 and ending at 1:1280. Results of ELISAs were analyzed using an automated plate reader.
In the process, twenty-two conjugate control wells were generated to monitor non-specific antibody binding (background) for the ELISAs. These control values were
averaged and used to calculate the standard deviation (SD). Two SD units were added to the average background value for each run which then served as the lower
limit for a positive result or measurable antibody response. For each penguin sample duplicate pair, the titer was defined as the reciprocal of the dilution at
which each duplicate was above the positive result cut-off or limit. The geometric mean of the titers for all six penguins of each group (saline, pCI, or
pCMV-Beta) was calculated for each day serum was collected. To illustrate our results, "Geometric mean ELISA titer" was plotted against "Days postinoculation"
(Figure 1).
Results show that penguins inoculated with the pCMV-Beta construct produced measurable anti-Beta-galactosidase antibodies when compared to
control animals. On Days 21, 28, and 42, there was a statistically significant difference (using single factor ANOVA) between the mean of log-transformed titers
of control birds (saline- or pCI-inoculated) versus birds vaccinated with the pCMV-Beta reporter plasmid (Figure 1). The occurrence of a rise in antibody titer
after "booster" vaccination with pCMV-Beta was not observed. These results demonstrate that African black-footed penguins, directly transfected with the gene
encoding Beta-galactosidase, are capable of mounting a humoral response against the in vivo expressed protein.
The portion of the study examining the humoral response of adult chain dogfish to IM plasmid vaccination is in progress. On Day O, two groups
of four dogfish each received either 50 micrograms of the control plasmid pCI, or 50 micrograms of the test plasmid pCMV-Beta, respectively. Serum was harvested
every 7 to 21 days for 16 weeks post-inoculation. "Booster" vaccines were given on Day 21. ELISA and Western blot assays are being used to screen the dogfish sera
for anti-Beta-galactosidase antibodies using anti-sandbar shark IgM. Although DNA vaccination has been investigated in several teleost
species1,2,10,11, plasmid vaccination in an elasmobranch species has not been reported; thus, data from this trial represent an exciting preliminary
look at the humoral immune response of elasmobranchs to nucleic acid vaccines.
This study demonstrated that African black-footed penguins are capable of immunologically responding to foreign proteins that are transferred
in the form of plasmid DNA. Analysis of immune response to DNA-mediated vaccination in chain dogfish is still underway. Once proven to induce an immune response
to well-characterized protein antigens, plasmid vaccination can be used as a tool for screening genes encoding potential protective antigens from various
bacterial, viral, or parasitic pathogens. Knowledge gained from this work will be integral in the future development of plasmid vaccines against specific
infectious diseases of penguins and chain dogfish in both captive and wild environments.
| Figure 1. | 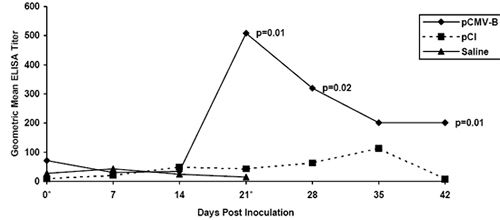
Geometric mean ELISA titer of anti-β-galactosidase antibody in six African black-footed penguins after immunization with a plasmid containing the gene for β-galactosidase (pCMV-B). |
|
| |
Plasmid was injected on days 0 and 21 (*). Penguins immunized with only saline diluent (Saline) and plasmid without
β-galactosidase gene sequences (pCI) were used as negative controls. Means of the log-transformed ELISA titers were used in a single-factor ANOVA to
generate the p-values on days 21, 28, and 42.
Acknowledgments
This project was supported in part by the Sea Research Foundation in Mystic, Connecticut. The authors wish to thank the generous members of
the Mystic Aquarium husbandry staff and of the Poet laboratory at the University of Georgia, whose time and efforts made this study possible. We are also grateful
to Dr. John Marchalonis at the University of Arizona for his time and the donation of anti-sandbar shark IgM. Special gratitude is extended to Ms. Tracey Schock,
a fine young researcher and newly-inducted ELISA whiz, without whom none of the results would have been generated. Finally, we are appreciative of the support
given by the International Association of Aquatic Animal Medicine for this project in the form of a Student Travel Award for the presenting author.
References
1. Anderson ED, DV Mourich, JC Leong, 1996. Gene expression in rainbow trout (Onchorhynchus mykiss) following intramuscular
injection of DNA. Mol Mar Biol Biotechnol 5:105-113.
2. Anderson ED, DV Mourich, SC Fahrenkrug, S LaPatra, J Shepherd, JC Leong, 1996. Genetic immunization of rainbow trout
(Onchorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol 5: 104-122.
3. Cardoso AI, M Blixenkrone-Moller, J Fayolle, M Liu, R Buckland, TF Wild, 1996. Immunization with plasmid DNA encoding for the
measles virus hemagglutinin and nucleoprotein leads to humoral and cell-mediated immunity. Virology 225:293-299.
4. Davis HL, ML Michel, RG Whalen, 1995. Use of plasmid DNA for direct gene transfer and immunization. Ann NY Acad Sci
772:30-39.
5. Fu TM, A Friedman, JB Ulmer, MA Liu, JJ Donnelly, 1997. Protective cellular immunity: cytotoxic T-lymphocyte responses against
dominant and recessive epitopes of influenza virus nudeoprotein induced by DNA immunization. J Virol 71:2715-2721.
6. Fynan EF, RG Webster, DH Fuller, JR Haynes, JC Snatoro, HL Robinson, 1993. DNA vaccines: Protective immunizations by parenteral,
mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA 90:11478-11482.
7. Hansen E, K Fernandes, G Goldspink, P Butterworth, PK Umeda, KC Chang, 1991. Strong expression of foreign genes following direct
injection into fish muscle. FEBS Lett 290:7376.
8. Kuklin N, M Daheshia, K Karem, E Manickan, BT Rouse, 1997. Induction of mucosal immunity against herpes simplex virus by plasmid
DNA immunization. J Virol 71:3 1383 145.
9. Manickan E, RJ Rouse, Z Ru, WS Wire, BT Rouse, 1995. Genetic immunization against herpes simplex virus: Protection is mediated by
CD4+ T lymphocytes. J Immunol 155: 259-265.
10. Poet SE, VV Burnley, 1997. DNA-mediated vaccination in channel catfish. Proceedings of the 28th Annual International Association
for Aquatic Animal Medicine, Harderwijk, The Netherlands 28:71.
11. Rahman A, N Maclean, 1992. Fish transgene expression by direct injection into fish muscle. Mol Mar Biol Biotechnol 1:286-289.
12. Ulmer JB, JJ Donnelly, SE Parker, GH Rhodes, PL Felgner, VJ Dwarki, SH Gromkowski, RR Deck, CM Dewitt, A Friedman, LA Hawe, KR
Leander, D Martinez, HC Perry, JW Shiver, DL Montgomery, MA Liu, 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein.
Science 259: 1745-1749.
13. Wang B, J Boyer, V Srikantan, K Ugen, L Gilbert, C Phan, K Dang, M Merva, MG Agadjanyan, M Newman, 1995. Induction of humoral and
cellular immune responses to the human immunodeficiency type I virus in nonhuman primates by in vivo DNA inoculation. Virology 211:102-112.
14. Wang B, KI Ugen, V Srilcantan, MG Agadjanyan, K Dang, Y Refaeli, AI Sato, J Boyer, WV Williams, DB Weiner, 1993. Gene inoculation
generates immune responses against immunodeficiency virus type 1. Proc Natl Acad Sci USA 90:4156-4160.
15. Whalen RG, C Leclerc, E Deriaud, R Schirmbeck, J Reimann, HL Davis, 1995. DNA-mediated immunization to the hepatitis B surface
antigen. Activation and entrainment of the immune response. Ann NY Acad Sci 772:64-76.
16. Xiang ZQ, SL Spitalnik, J Cheng, J Erikson, B Wojczyk, HCJ Ertl, 1995. Immune responses to nucleic acid vaccines against rabies virus.
Virology 209:569-579.