N. Clancey
Atlantic Veterinary College, Pathology & Microbiology, Charlottetown, PEI, Canada
Introduction
Cytology is a minimally invasive, relatively inexpensive, powerful clinical tool allowing for rapid and sometimes critical information. This information may be definitive or may only provide partial information, but often will narrow differential lists or provide justification for additional diagnostic procedures. Regardless, experience is necessary to make sound conclusions and to avoid over- or under-interpretation.
Indications for cytology samples vary. If a tissue or fluid is suspected to be abnormal and a cytologic sample can be safely collected, cytology is encouraged as an initial diagnostic test. Cytology is commonly used to evaluate skin lesions, enlarged lymph nodes and a variety of fluid samples such as airway washes, cavity effusions, joint and cerebrospinal fluids. With the aid of imaging modalities, particularly ultrasound guidance, cytology is also routinely used to assess internal organs. Collection methods, sample preparation and general suggestions are discussed further under Diagnostic Cytology: Optimizing Sample Quality for Improved Results.
Cytological evaluation begins with a well-maintained and properly set up microscope, including Köhler illumination. Köhler illumination generates even lighting and sharp resolution of sample features. Websites of most microscope manufacturing companies and online videos demonstrating Köhler illumination procedures are available.
Gross examination of slides for excessive blood, large cellular clumps, defects and greasiness can help assess sample quality and where to focus attention once viewed microscopically. A systemic approach should be employed to avoid corner-cutting and limit errors.
Step 1) Review the entire slide at low magnification to assess sample quality. Ask yourself:
- Are sufficient cell numbers present?
- Are the cells well-preserved? Poorly preserved? Are they intact?
- Has there been adequate spreading of cells? Adequate staining of cells?
- Are normal cells anticipated, such as columnar ciliated epithelial cells in transtracheal washes?
- What areas are worthy of further evaluation at higher magnification?
Step 2) Assuming sample quality is adequate, assess cellular arrangement at lower magnifications. Are cells found only as individual cells? Are tight cohesive clusters of cells present? These findings will aid in determining cell populations and cell origins at higher magnifications. At higher magnification, classify cells into big categories. Are the cells inflammatory, non-inflammatory or a combination?
Step 3a) If cells are inflammatory, determine the type(s)—neutrophils, histiocytes/macrophages, lymphocytes, plasma cells, eosinophils, basophils or mast cells. If mast cells predominate, the lesion is almost certainly a mast cell tumour, not an inflammatory lesion.
Step 3b) If cells are non-inflammatory, determine the type(s)—focus on the broad categories of epithelial, mesenchymal, round cell and neuroendocrine. These categories do not necessarily reflect cell origin or function. Rather, they are based on cytomorphological characteristics, including their general association with each other. Also evaluate for normal cells (are any seen and/or anticipated?) and for cells thought to be hyperplastic or dysplastic.
Step 4a) If inflammation is diagnosed, a search for possible infectious organisms is warranted. The presence of degenerate neutrophils often provides a diagnostic clue and helps warrant continued search to identify intracellular bacteria. The type of inflammation will guide possible infectious considerations.
Step 4b) If neoplasia is diagnosed, the next logical information a clinician will want is whether the neoplasm is benign or malignant. This is determined by evaluating cellular criteria of malignancy. Generally, nuclear criteria are more reliable than cytoplasmic criteria for estimating malignancy potential. Recognition of 3 or more malignant criteria in a high percentage of tumour cells is often cited to diagnose malignancy. However, exceptions certainly exist. It is not always possible to determine whether a cell population is benign or malignant on cytology alone as not all neoplastic behaviour correlates well with cellular atypia or lack thereof.
Figure 1
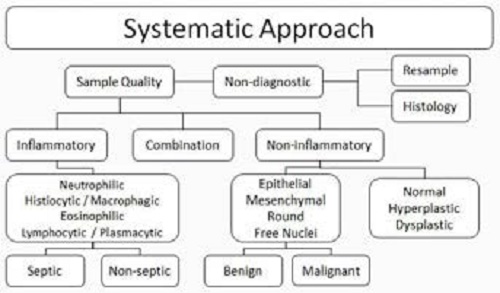
Inflammatory conditions are classified based on the predominating cell type present.
Neutrophilic inflammation: This is diagnosed when neutrophils comprise >70–85% of cellularity. Neutrophilic inflammation can be acute or chronic and be due to various infectious and non-infectious conditions. Evaluating for neutrophil degeneration can often help narrow considerations. Non-degenerate neutrophils are present in relatively non-toxic environments such immune-mediated disorders, sterile foreign body lesions and neoplastic conditions. Degenerate neutrophils have swollen, pale staining nuclei, supporting rapid death in a toxic environment. Neutrophil degeneration occurs most commonly due to bacterial toxins, but other causes are possible. Neutrophils chronically present in fluid environments such as urine or body cavity effusions may imbibe fluid and have features mimicking degeneration. Conversely, even if neutrophils are non-degenerate, a bacterial infection should still be considered as bacteria may be present in low numbers or they may only produce small amounts of toxin.
Mixed inflammation: If neutrophils comprise ~50–70% of cellularity and remaining cells are mononuclear (macrophages, lymphocytes, plasma cells), mixed (neutrophilic and mononuclear cell) inflammation is diagnosed. This is also called pyogranulomatous or chronic-active inflammation. We look for degeneration of neutrophils, infectious agents and foreign material. This type of inflammation is associated with foreign body reactions, fungal and mycobacterial infections, panniculitis, lick and sterile granulomas and various chronic tissue injuries.
Histiocytic/macrophagic inflammation: This type of inflammation is diagnosed when macrophages comprise >50% of cellularity. It is typical of low-grade irritation and is often associated with systemic mycoses and sterile foreign bodies. It may also represent resolving inflammation of a previously more active lesion. Presence of inflammatory giant cells and epithelioid macrophages support granulomatous inflammation. When observed, these cells strongly support a persistent cause, such as a systemic mycotic infection or a sterile foreign body reaction.
Eosinophilic inflammation: This is typically diagnosed when eosinophils comprise >10% of cellularity. Eosinophils may be the predominant cell type in select lesions (e.g., eosinophilic granulomas) but are often found in association with other inflammatory cells. Eosinophilic inflammation is typically associated with allergic hypersensitivity reactions, fungal infections, parasitic migrations, mast cell tumours and other select neoplasms.
Non-inflammatory cells may be normal, hyperplasic, dysplastic or neoplastic, and effort should be made to place them into one or more of the following classic cytomorphological categories.
Epithelial cells: These tend to exfoliate in tight cohesive clusters or sheets, although individual epithelial cells can certainly be seen. When in clusters or sheets, cells bind by distinct tight junctions (desmosomes), often providing distinct alignment and abutting of cells to each other. This helps give epithelial cells distinct cellular margins. Palisading, acinar, lobular and trabecular arrangement of cells are all typical findings. Although size can vary, epithelial cells are often large, round to polygonal cells with well-defined cell borders. Nuclei are usually round to plump ovoid in shape.
Mesenchymal cells: Compared to epithelial cells, mesenchymal cells are poorly exfoliative, often resulting in poorly cellular samples. When present, cells tend to exfoliate as individual cells or in variably sized clumps or clusters. Unlike tightly arranged epithelial cell clusters, aggregates of mesenchymal cells are loosely cohesive and often have storiform arrangement. Bright pink (with Wright-Giemsa staining), extracellular, granular to amorphous material representative of extracellular matrix may be seen closely associated with cells. Mesenchymal cells are classically stellate to spindloid but may be ovoid. They tend to lack distinct cell margins and have wispy cytoplasm that often trails to fine points or blends with the background. Nuclei are classically elliptical or elongate but may be ovoid or round.
Round cells: Round cell lesions are often rewarding as they readily exfoliate providing highly cellular samples. Cells exfoliate individually but may be present in clumps. Single cells have distinct cellular margins and are round in shape. They tend to be smaller than epithelial cells with round, ovoid to reniform or indented nuclei. Application of malignant criteria to round cell tumours may not be as helpful as for epithelial and mesenchymal cell tumour. This is because biological behaviour appears more specific to the round cell tumour type than degree of morphological atypia.
Neuroendocrine (free nuclei) cells: Cells from these tissues often exfoliate easily and in loosely cohesive sheets with many free nuclei present. Occasional clusters may have distinct cell outlines. When intact, cells are generally round to mildly polygonal. Nuclei are typically round to indented. Cells from neoplastic lesions often have no to minimal anisokaryosis, yet these tumours often have aggressive behaviour.
Concurrence of inflammatory and non-inflammatory cells warrants special attention as dysplastic or reactive changes to epithelial and mesenchymal cells caused by inflammation often mimic malignant criteria. Urothelial, squamous and respiratory epithelia, and mesothelium are prone to appear malignant due to dysplastic or reactive features, requiring experience to differentiate. Similarly, reactive fibroblasts associated with significant inflammation can easily have marked cytologic atypia and potentially be misdiagnosed as a sarcoma with secondary inflammation to the untrained eye.
References
1. Barger AM, MacNeill A. Small Animal Cytologic Diagnosis. 1st ed. Boca Raton: CRC Press; 2017.
2. Cian F, Freeman K. Veterinary Cytology. 2nd ed. Boca Raton: CRC Press; 2017.
3. Raskin RE, Meyer D. Canine and Feline Cytology: A Color Atlas and Interpretation Guide. 3rd ed. St. Louis: Elsevier; 2016.
4. Valenciano AC, Cowell RL. Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat. 4th ed. St. Louis: Elsevier; 2013.
5. World Small Animal Veterinary Association. Szladovits B. Cytology in Practice. 2014 [internet]. Serbia: WSA-VA; 2014. Available from: www.sasap.org.rs/download/pf_1423495947_fe22.pdf.