State-of-the-Art Lecture: Advanced Lung Ultrasound: Subpleural Consolidations and Bronchograms
S. Boysen
Faculty of Veterinary Medicine, Veterinary Clinical and Diagnostic Sciences, University of Calgary, Calgary, AB, Canada
Introduction
Thoracic veterinary point of are ultrasound (VPOCUS) has evolved greatly over the last several years. The initial thoracic FAST study by Lisciandro et al. in 2008 described 4 sites on the thorax (bilateral chest tube site, bilateral pericardial site) and was designed to detect pathology in the pleural space (pneumothorax, pleural effusion) and the pericardial space (pericardial effusion). The study was not originally intended to assess lung pathology. With the development and validation of several human lung ultrasound protocols, a number of veterinary small animal lung ultrasound protocols have recently been published in dogs (see below). Only one study has published normal lung findings using lung ultrasound in cats.
Different Lung Ultrasound Protocols Published in Dogs
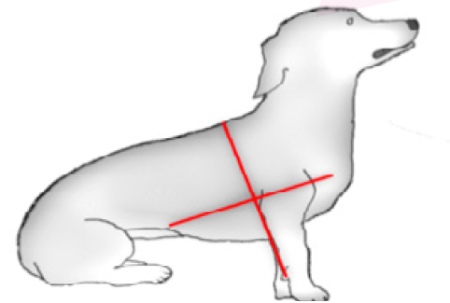
The Radamacher protocol (2014) divides each hemithorax into four quadrants: craniodorsal, cranioventral, caudodorsal, and caudoventral. The sixth intercostal space was determined as the limit between the cranial and caudal areas and the elbow as the limit between dorsal and ventral. Patients are scanned in sitting or standing.
Radamacher protocol (2014)
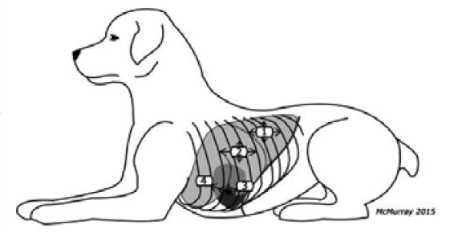
The Lisciandro Vet BLUE (2014) places the prove stationary and horizontally at the caudodorsal lung lobe region. The starting point for Vet BLUE is the same point referred to as the chest tube site in Thoracic FAST. Similarly, lung is observed at the perihilar, middle, and cranial lung lobe regions. Patients are scanned in sternal or standing.
Lisciandro Vet BLUE protocol (2014)
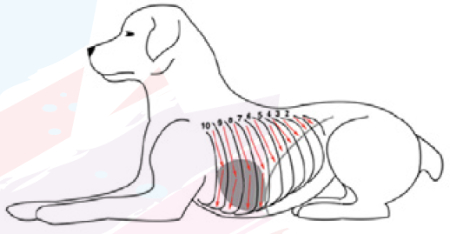
In the Vezzosi protocol (2016), each hemithorax was examined by sliding the probe from dorsal to ventral, examining all intercostal spaces. Dogs are positioned in standing p and manually restrained.
Vezzosi protocol (2016)
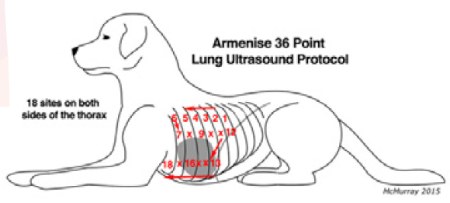
The Armenisie protocol (2018) is a horizontal sliding technique. Starting from the upper caudal dorsal 9th intercostal space and moving cranially, examining each intercostal space up to the 4th intercostal space, followed by the middle third of the thorax moving caudally one intercostal space at a time from the 4th to the 9th intercostal spaces, and finally the ventral third of the thorax moving cranially one intercostal space at a time from the 9th to the 4th intercostal spaces.
Armenisie protocol (2018)
Principles of Lung Ultrasound
The interface between soft tissue structures (e.g., internal thoracic muscles and pleura) and air (air filled lung) results in 99.9% of the ultrasound beam being reflected, rendering aerated lung impenetrable to ultrasound. Therefore, any structures below the visceral pleura are not sonographically visible when the lung is filled with air.
However, it is possible to detect lung pathology through the identification of lung surface artifacts when the lung is aerated (B lines) and consolidated lung regions when air is no longer present within the lung (bronchograms, consolidation).
Key Point
Lung ultrasound varies from the detection of true structure visualization in states where there is less than 5% air in the lung (e.g., consolidation) to the detection of artifacts in conditions where the lung is predominantly aerated (10–95% aerated lung). To be detected by lung ultrasound, it is important to remember that these pathologies must reach the lung surface.
The lung appearance, from healthy aerated lung to consolidation with conditions such as bronchopneumonia includes progression from the glide sign and A lines (healthy lung), to the glide sign with occasional B lines (mild wet lung), to coalescing B lines with or without pleural irregularities (markedly wet lung), to a glide sign with a shred sign (partial lung consolidation) to a glide sign with hepatization or tissue sign (fully consolidated from one pleural surface to the other). It is important to note that consolidation can occur as the result of simply atelectasis (with otherwise normal underlying lung tissue) to significant pathology such as consolidation associated with aspiration pneumonia.
Criteria to Diagnose Alveolar Consolidation
Alveolar Consolidation
Consolidated lung can appear as a tissue sign, shred sign, subpleural nodules or even thromboemboli.
Tissue-like sign: When the lungs become highly fluid filled, they can resemble the echogenic appearance of the liver. This is termed hepatization. When extensive the pleural line on the opposite side of the lung becomes visible. Consolidation is often accompanied by pleural effusion.
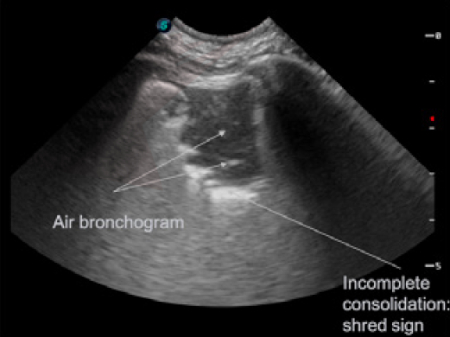
Shred sign: In cases of less extensive consolidation when there is still significant air in the lung echo poor areas are noted, which appear as though someone has “taken a chunk out of the lung surface”. These less extensive areas of consolidation are bordered by aerated lung with the presence of a glide sign and B lines.
Figure A
Air bronchograms: Air bronchograms appear as white punctate (end on) or linear (longitudinal) structures contained within consolidated lung. They can be classified as static (no movement during respirations) and dynamic (move with respirations). Air bronchograms help distinguish between consolidated and atelectatic lung; dynamic bronchograms are more likely to be seen with pneumonia/infection while static bronchograms are more often seen with atelectasis. Fluid bronchograms can also be seen in severe lung pathology.
Figure B
Pulmonary thromboembolism (PTE): Human studies have demonstrated that lung ultrasound is very sensitive but not specific for detecting pulmonary embolism. Sonographically, PTE is a type of consolidation, often between 0.5 and 3 cm in size, forming well-demarcated echo-poor triangular, rounded or polygonal consolidations in the absence of air inclusions/bronchograms.
They tend not to have surrounding inflammatory changes (Inflammatory changes = b-lines) and are more likely to be PTE when located in more than 2 locations, particularly if pleural fluid also present.
They demonstrate an absence of blood flow when using doppler. They can easily be confused for nodules. Because they are not specific, they must be interpreted in light of clinical signs!
Atelectasis: In human medicine, pathologic lung consolidation is often difficult to differentiate from atelectasis using ultrasound. In the presence of large pleural effusions, then compression atelectasis should be considered (figure 12). If only small pleural effusion is present than consolidation is more likely. Draining pleural effusion should re-inflate atelectatic lung, and change its sonographic appearance, while consolidated lung will often remain consolidate following removal of pleural effusion. Dynamic bronchograms are more likely to be seen with pneumonia/infection while static bronchograms are more often seen with atelectasis.
Lung surface characteristics: There is emerging evidence in the human literature that the surface of the lung (smooth) vs. irregular may help differentiate cardiogenic (smooth surface) from non-cardiogenic (irregular lung surface with subpleural lesions) causes of AIS.
Criteria to Diagnose Consolidation
- Abnormal pattern should be in thorax (should be differentiated from the liver or spleen)
- Should arise from the pleural line
- There should be a tissue like pattern (similar to liver echotexture)
- Anatomic boundaries must be present: Superficial boundary of consolidation
- At the pleural line in the absence of pleural effusion
- At the deep boundary of a pleural effusion if effusion present
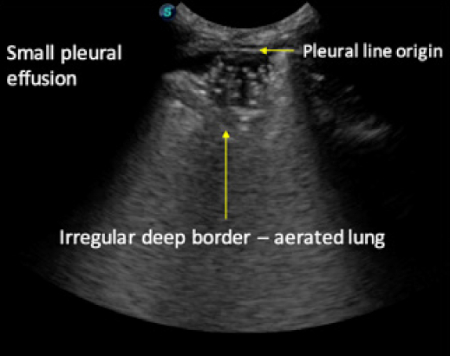
- Deep boundary of the consolidation may be irregular (aerated lung boundary: figure 6) or regular (if whole lobe is consolidated: figure 7)
- Where consolidation fails to reach the deep border of the lung and comes in contact with air an irregular consolidation/air interface is created referred to as a “shred sign”
- Where consolidation extends through the entirety of the lung, from one surface to the other, hepatization or a tissue sign is seen
Summary
In human medicine, studies demonstrate that lung ultrasound has a higher diagnostic accuracy than physical examination and chest radiography combined. In general, human lung ultrasound can be used to detect pneumothorax, pleural effusion, alveolar interstitial syndrome (AIS) irregular lung surfaces, small subpleural consolidations (<0.5 cm), larger subpleural consolidated lung lesions (hepatic sign, shred sign >3 cm), air bronchograms, subpleural pulmonary nodules, and has been used to detect pulmonary thromboembolism that reaches the lung surface. In veterinary medicine, although a lot of clinicians are applying veterinary point of care lung ultrasound protocols to help diagnose the above conditions, only pneumothorax, pleural effusion and AIS have been published using point of care ultrasound techniques. The differential diagnosis for subpleural consolidation should be considered in light of the entire clinical picture, history and to some degree, the shape and distribution/severity of the consolidation.