Cetacean Anesthesia: A Review of 34 General Anesthesia Events, Lessons Learned, and Future Plans
Abstract
Although applied infrequently, anesthesia of cetaceans has become an acceptable risk. Historical failures and limited experience led to many myths and misconceptions about anesthesia of cetaceans. As well, the relative difficulty with invasive support and monitoring had caused hesitation in performance of medically necessary invasive procedures, only increasing the probability of negative outcomes and further perpetuating these misconceptions. A coalition-of-the-willing was built at the IAAAM conference over the recent past, and IAAAM members have continued efforts to update surgical and anesthetic management of cetaceans.1-23 The following is evidence of their strong efforts.
We present here a series of anesthesia events involving bottlenose dolphins (Tursiops truncatus, Tursiops truncatus gilli, and Tursiops truncatus aduncus) and Pacific white-sided dolphins (Lagenorhynchus obliquidens). Currently, benzodiazepines alone (diazepam, midazolam), or in combination with opioids (butorphanol, meperidine) are used for preanesthetic sedation. Intravenous access via ultrasound-guided cannulation of the lateral subcutaneous caudal vein (LSCV) has become routine. The injectable anesthetic agent, propofol, is used for rapid sequence induction allowing manual orotracheal intubation by rostral luxation of the elongated epiglottal and cricoarytenoid cartilages or “goosebeak.” The inhalation anesthetic agents, isoflurane and sevoflurane, are used reliably for maintenance of anesthesia. Though statistical analysis cannot easily be applied, the median subject (22-year-old and 187.5 kg) minimally received either 0.25 mg/kg diazepam PO or 0.08 mg/kg midazolam IM for sedation, followed by median dosages of 2.93 mg/kg of propofol for induction, and an end-expired sevoflurane concentration of 1.0% to 2.8% for maintenance of anesthesia, for a total of 96 minutes. Conventional Controlled Mechanical Ventilation (CMV) was applied with variable success, as well as the alternative mode, Apneustic Anesthesia Ventilation (AAV), currently undergoing clinical trials. Anesthetic monitoring and vascular access are complicated by anatomic specializations; however, direct arterial blood pressure monitoring was feasible via cannulation of the median artery of the pectoral flipper. The mean arterial blood pressure (MAP) of those measured was initially low (55 mm Hg), but appropriate support improved values to median highs of 84 mm Hg, intraoperatively. Such support involved reducing anesthetic where possible, balancing the anesthetic technique with opioids, volume expanding with IV crystalloid fluids, and use of inotropes (ephedrine, 0.05–0.1 mg bolus IV; dobutamine, 0.2–2.0 mcg/kg/min IV) to increase contractility, cardiac output, and blood pressure. All MAP improved upon discontinuation of anesthesia for recovery (131 mm Hg). Vasopressors (phenylephrine, 1–3 mcg/kg/min IV) were also used in certain instances along with the inotropes.
Upon entering recovery, the median body temperature was 35.5°C, and successful extubation required 61 minutes (including those requiring re-intubation and additional time), thus necessitating continued physiologic monitoring and support in recovery. Common concerns include endotracheal intubation and extubation, body positioning and padding, body temperature management, vascular access, blood pressure, variable response to opioids, pharmacokinetics of anesthetic agents, ventilation and acid-base derangements. New modes of ventilation, imaging techniques, and advanced non-invasive monitoring methods will advance the care of these species under general anesthesia, and lessons learned with plans for improving management will be presented here in brief.
Table 1
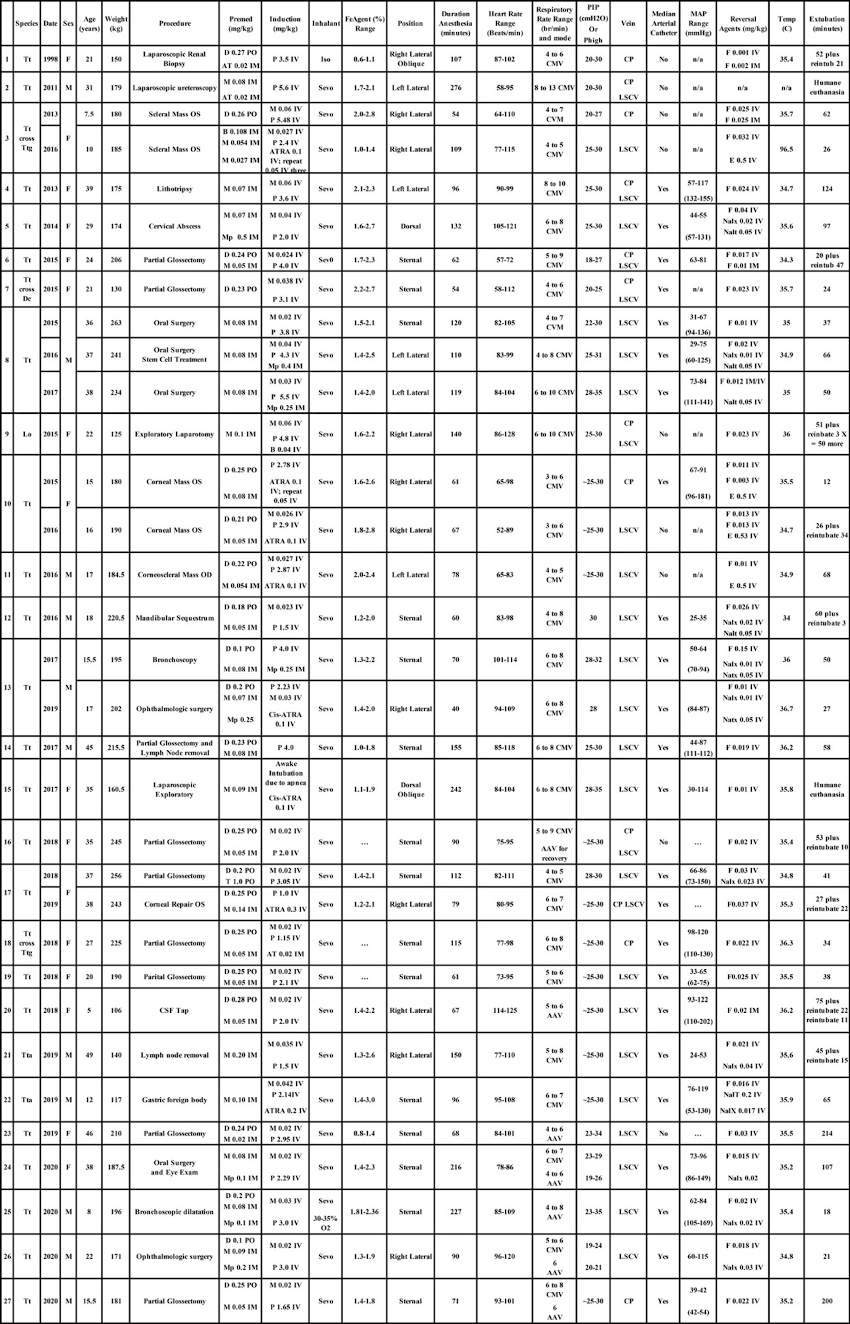
Thirty-four anesthesia events involving 27 different individual small cetaceans. Bottlenose dolphin Tt = Tursiops truncatus; Ttg = Tursiops truncatus gilli, and Tta = Tursiops truncatus aduncus; Common dolphin Dc = Delphinus capensis; Pacific white-sided dolphin Lo = Lagenorhynchus obliquidens; Cumulative dosages of the drugs: AT = Atropine; ATRA = Atracurium; B = Butorphanol; D = Diazepam; E = Edrophonium; F = Flumazenil; Iso = Isoflurane; Mp = Meperidine; T = Tramadol; M = Midazolam; Nalx = Naloxone; Nalt = Naltrexone; Propofol = P; Sevo = Sevoflurane. PO = per os; IM = intramuscular; IV = intravenous. FeAgent = fraction expired inhalation anesthetic. Anesthesia duration was documented as the time from injection of IV induction agent until the vaporizer was turned off and the anesthetic circuit was flushed with oxygen to minimize inhalation anesthetic for the recovery phase to begin. CMV = conventional Controlled Mechanical Ventilation; AAV = Apneustic Anesthesia Ventilation; PIP = Peak Inspiratory Pressure; Phigh = plateau pressure or pressure high; LSCV = Lateral subcutaneous caudal vein; CP = caudal peduncle vein; MAP = mean arterial blood pressure (note: parenthesis indicate MAP in semi-conscious and spontaneously ventilating subject during recovery phase with arterial catheter maintained until extubation). Time of detection of hypotension generally coincided with the time of placement of the arterial catheter. Reversal agent dosages were total dosages that include repeat dosing. Temp = Temperature as measured at end of anesthesia. Extubation = Time to extubation was measured from the point the anesthetic circuit was initially flushed with oxygen to minimize inspired inhalation anesthetic until extubation. (Reproduced with permission from Bailey J. Innovative Veterinary Medicine Inc.: 101 Marketside AVE, STE 404-402, Ponte Vedra, FL 32081-1541, 2021).
Figure 1
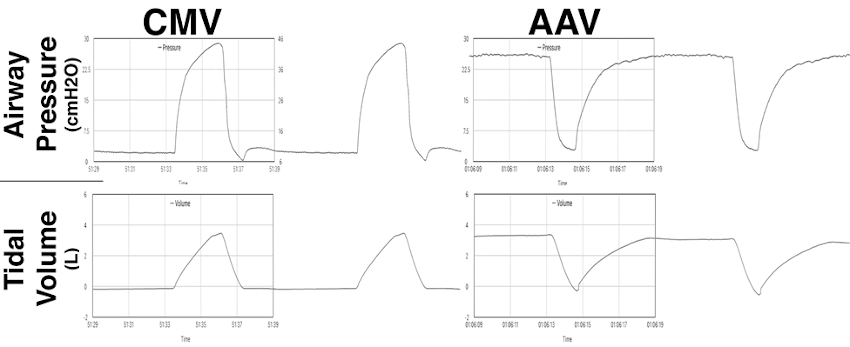
CMV vs AAV ventilation mode spirometry waveforms. Example of the change in airway pressure and lung volume during the Controlled Mechanical Ventilation mode (CMV; left column) and during the Apneustic Anesthesia Ventilation mode (AAV; right column) when applied to the same 190 kg adult bottlenose dolphin. In applying CMV the airway pressure increases from residual or ambient pressure to the peak pressure of 29 cm H2O (range: 20 to 30 cm H2O in anesthetized dolphins) to generate a 3.5-liter tidal volume. In contrast, when applying AAV the pressure is released from the selected upper level (Phigh) to lower level (Plow). In this instance, the Phigh is 26 cm H2O and the Plow is 2 cm H2O, generating the same 3.5 liter tidal volume. CMV increases up from the functional residual capacity (FRC) or lower over time, where AAV decreases from the selected elevated plateau value, down to a level slightly above FRC. Following the release time of AAV, the lung volume is rapidly re-established by reapplication of Phigh. (Reproduced with permission from Bailey J. Innovative Veterinary Medicine Inc.: 101 Marketside AVE, STE 404-402, Ponte Vedra, FL 32081-1541, 2021).
Acknowledgements
The institutions driving these recent advancements in anesthetic care of cetaceans are not only large, such as the U.S. Navy Marine Mammal Program, National Marine Mammal Foundation, SeaWorld USA, and Vancouver Aquarium, but also relatively smaller, as well as international facilities. Many individuals of the marine mammal community, and other interested personages, have contributed to the development of cetacean anesthetic techniques over the years—and so many more have worked to advance the methodology by contributing to the cases discussed here. Acknowledgment and gratitude go to all, but their names are far too numerous to list here. However, there is one individual who pioneered the methods of dolphin anesthesia we use today, and has mentored, guided, or in some way promoted all of our efforts. His name is Dr. Sam H. Ridgway—anchor and concatenator of the past, present, and future of cetacean anesthesia. Thank you, Sam.
Literature Cited
1. Ridgway SH. 1971. Some Observations on the Physiology and Pathology of Aquatic Mammals. IAAAM 3rd Annual Conference Proceedings, Guelph, Ontario, Canada.
2. Dold C, Van Bonn W, Smith C, Wong S, Jensen E, Ridgway S. 2005. Addressing Challenges of Anesthesia Monitoring and Support for the Bottlenose Dolphin (Tursiops truncatus). IAAAM 36th Annual Conference Proceedings, Seward, AK.
3. Dold C, Jensen E, Stetter M, Hendrickson D. 2011. Renewed Steps Toward Minimally Invasive Surgery in Bottlenose Dolphins (Tursiops truncatus). IAAAM 42nd Annual Conference Proceedings, Las Vegas, NV; Pp. 102–103.
4. Dold C, Jensen E, Stetter M, Hendrickson D. 2012. Renewed Steps Toward Minimally Invasive Surgery in Bottlenose Dolphins (Tursiops truncatus). IAAAM 43rd Annual Conference Proceedings, Atlanta, GA; Pp. 226–227.
5. Dover SR, Kolata R, Walsh MT. 1998. The Development of Laparoscopic Techniques for Use in Marine Mammals. IAAAM 29th Annual Conference Proceedings, San Diego, CA.
6. Dover SR, Beusse D, Walsh MT, McBain JF, Ridgway S. 1999. Laparoscopic Techniques for the Bottlenose Dolphin (Tursiops truncatus). IAAAM 30th Annual Conference Proceedings, Boston, MA.
7. van Elk CE, van Bonn W, van Loon T, Voermans M, Vogel H. 2010. Surgical Intervention in a Bottlenose Dolphin with Chronic Arthritis of the Right Shoulder Joint. IAAAM 41st Annual Conference Proceedings, Vancouver, British Columbia, Canada.
8. Coisman J, Walsh MT, Wellehan J, Ellison GW, Jensen ED, Hendrickson DA, Dold C. 2013. Looking Through the Portal: Laparoscopy in Small Cetaceans. 2013. IAAAM 44th Annual Conference Proceedings, Sausalito, CA; Pp. 300.
9. Meegan J, Smith CR, Johnson SP, Sur RL, Hendrickson D, L’Esperance J, Bailey JE, Gomez FM, Lutmerding BA, Hoh C, Schmitt TL, Le-Bert CR, Weisse C, Berent A, Ridgway SH, Jensen ED. 2012. Medical and Surgical Management of a Male Bottlenose Dolphin (Tursiops truncatus) with Chronic Severe Bilateral Renal Nephrolithiasis. IAAAM 43rd Annual Conference Proceedings, Atlanta, GA; Pp. 222–223.
10. Schmitt T, Nollens HH, Esson DW, St. Leger J, Bailey J. 2014. Superficial Keratectomy and Cryosurgery of a Limbal Melanoma Under General Anesthesia in a Bottlenose Dolphin (Tursiops truncatus gilli). IAAAM 45th Annual Conference Proceedings, Gold Coast, Australia.
11. Meegan J, Cotte LS, Ivančić M, Thornton JJ, Deming AC, Bailey JE, Emory-Gomez FM, Costidis A, Wisbach GG, Hendrickson D, Weiss JS, Gallus DP, Smith CR, Jensen ED. 2015. Medical and Surgical Management of a Chronic Cervical Abscess in a Bottlenose Dolphin (Tursiops truncatus). IAAAM 46th Annual Conference Proceedings, Chicago, IL.
12. Smith C, Meegan JM, Bailey J, Scott GN, Sur R, L’Esperance J, Ivančić M, Cotte LS, Cendejas V, Sakhaee K, Ridgway SH, Jensen ED. 2015. Case Report: Surgical Management of a Partial Ureteral Obstruction Under General Anesthesia in a Geriatric Bottlenose Dolphin. IAAAM 46th Annual Conference Proceedings, Chicago, IL.
13. Ivančić M, Johnson S, Costidis AM, Renner MS. 2015. A Technique for Ultrasound-Guided Catheterization of a Peripheral Vein in the Bottlenose Dolphin (Tursiops truncatus). IAAAM 46th Annual Conference Proceedings, Chicago, IL.
14. Haulena M, Rosenberg J, Bailey J, Hendrickson D, Ivančić M, Raverty S. 2016. General Anesthesia and Exploratory Laparotomy in a Pacific White-Sided Dolphin (Lagenorhynchus obliquidens). IAAAM 47th Annual Conference Proceedings, Virginia Beach, VA.
15. Meegan JM, Woody AD, Bailey JE, Jensen ED, Gomez FM, Garman LS, Lutmerding BA, Smith CR, Nau M, Ivančić M, Ridgway SH. 2016. Surgical Management of Osteomyelitis and Dental Disease in a Bottlenose Dolphin (Tursiops truncatus) Using General Anesthesia. IAAAM 47th Annual Conference Proceedings, Virginia Beach, VA.
16. Yanagisawa M, Yamashita K, Tamura J, Endo Y, Koie H, Izumisawa Y, Ueda K. 2016. Surgical Treatment of Osteomyelitis in Indo-Pacific Bottlenose Dolphin (Tursiops aduncus). IAAAM 47th Annual Conference Proceedings, Virginia Beach, VA.
17. Bailey JE. 2016. Cetacean Anesthesia: A Review of 10 Clinical Anesthesia Events, Lessons Learned and Future Plans. IAAAM 47th Annual Conference Proceedings, Virginia Beach, VA.
18. Johnson SP, Ferrara S, Hill LD, Jensen E, Lutmerding B, Ridgway S. 2010. Ultrasound-Guided Percutaneous Transhepatic Catheterization in the Bottlenose Dolphin (Tursiops truncatus). IAAAM 41st Annual Conference Proceedings, Vancouver, British Columbia, Canada.
19. Ivančić M, Bailey JE, Costidis AM. 2014. Technique for Ultrasound-Guided Arterial Catheter Placement in the Pectoral Flipper of the Bottlenose Dolphin (Tursiops truncatus). IAAAM 45th Annual Conference Proceedings, Gold Coast, Australia.
20. Walsh M, Reidarson T, McBain J, Dalton L, Gearhart S, Chittick E, MS, Schmitt T, Dold C. 2006. Sedation and Anesthesia Techniques in Cetaceans. AAZV Conference Proceedings; Pp. 237–239.
21. Bailey J. 2013. Cetacean Sedation and Anesthesia: Review and Update. AAZV Conference Proceedings; Pp. 17.
22. Doescher BM, Pawloski J, Bailey JE. 2018. General Anesthesia to Facilitate the Surgical Resection of an Oral Squamous Cell Carcinoma in a Geriatric Bottlenose Dolphin (Tursiops truncatus). IAAAM 49th Annual Conference Proceedings, Long Beach, CA.
23. Schmitt TL, Knych HK, Mama KR, Nollens HH, Croft L, DiRocco S, Erlacher-Reid C, Bailey JE. 2018. Plasma Concentrations and Disposition of Propofol in Bottlenose Dolphins (Tursiops truncatus) During General Anesthesia. IAAAM 49th Annual Conference Proceedings, Long Beach, CA.
24. Bailey JE, Dold C, Ridgway S. 2021. Cetacean Anesthesia. In: West G, Heard H, Caulkett N, editors. Zoo Animal & Wildlife Immobilization & Anesthesia. 3rd ed. Ames (IA): Blackwell Publishing. In Press.