Apneustic Anesthesia Ventilation: A Modified Approach for Marine Mammal Ventilatory Support
Abstract
Anesthesia-induced respiratory depression of large, breath-holding marine mammals is a well-known, yet poorly understood, anesthetic risk with the potential for significant complications.1-3 Morbidity under general anesthesia may include ventilation-perfusion inequality, hypercarbia, and right-to-left intrapulmonary shunting with resultant hypoxemia. Adequate ventilatory support is, therefore, critical in the successful management and outcome of marine mammals under anesthesia.
In 1964, the limitations of conventional controlled mechanical ventilation for marine mammal application were addressed through the application of apneustic plateau ventilation (APV). This ventilation mode employs an elevated baseline pressure followed by a transient drop in airway pressure to 0 cmH2O.4-5 It may also promote progressive atelectasis, decreased lung compliance, ventilation-perfusion derangements, and progressive hypoxemia while under mechanical ventilation.5 In 2014, the U.S. Navy Marine Mammal Program identified the need to improve upon the APV modifications accomplished in the 1960s. With previous APV successes and limitations in mind, the apneustic anesthesia ventilation (AAV) technique was proposed for mechanical ventilation of marine mammals.6 In this mode, the rapid, transient drop in airway pressure is such that lung volume is at or above functional residual capacity in order to limit atelectasis and improve lung compliance. AAV may thus provide lower mean intrathoracic pressure, as well as improved venous return, cardiovascular performance, and ventilation-perfusion matching compared to other ventilation techniques. Here, we provide a review of ventilation mechanics, as well as preliminary evaluation of AAV in a variety of large marine mammal species and discuss the potential for future applications.
Figure 1. Graphical representation of conventional mechanical ventilation (CMV) and apneustic anesthesia ventilation (AAV) superimposed on individual pressure-volume curves
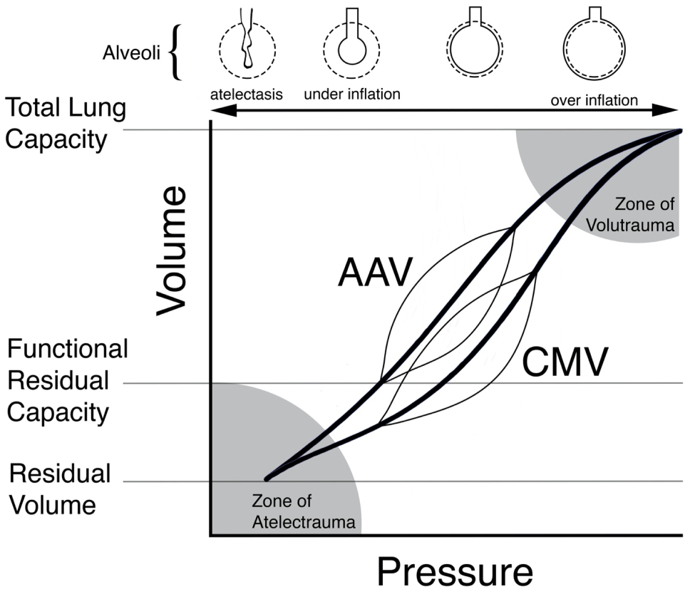
The AAV mode of ventilation preserves lung architecture and compliance. On the contrary, compliance decreases over time as atelectasis develops during the CMV mode of ventilation, shifting the compliance curve to the right. To achieve the same tidal volume, higher pressures are required during CMV.7
Acknowledgements
The authors thank the many physicians, veterinarians, veterinary technicians, and animal handlers and trainers, from a variety of veterinary and medical institutions, who have contributed to the success of this project to date. From University of Missouri’s College of Medicine: Dr. Alex Bukoski, Dr. Kelsey Fisher, Dr. John Dodam; The Marine Mammal Center: Dr. Shawn Johnson, Dr. Cara Field, Dr. Emily Trumbull; SeaWorld Adventure Parks: Dr. Hendrik Nollens, Dr. Jenn Russell, Dr. Stacy DiRocco, Dr. Chris Dold; National Marine Mammal Foundation: Dr. Forrest Gomez, Dr. Cynthia Smith, Dr. Jenny Meegan, Dr. Barb Linnehan, Dr. Abby McClain, Celeste Parry, Brittany Novick, Kevin Carlin, Veronica Cendejas, Mark Baird, Sacha Stevenson, Erin Brodie, Jammy Eichmann; US Navy Marine Mammal Program: CPT Kyle Ross, SPC Perron, Dr. Mark Xitco, Dr. Eric Jensen. Funding for this work is supported through the Office of Naval Research STTR Award N00014-14-P-1200.
*Presenting author
Literature Cited
1. Dold C, Ridgway S. 2014. Cetaceans. In: West G, Heard D, Caulkett N, editors. Zoo animal and wildlife immobilization and anesthesia. 2nd ed. Ames (IA): Blackwell Publishing. p 679–691.
2. Haulena M. 2014. Otariid Seals. In: West G, Heard D, Caulkett N, editors. Zoo animal and wildlife immobilization and anesthesia. 2nd ed. Ames (IA): Blackwell Publishing. p 661–672.
3. Haulena M, Schmitt T. 2018. Anesthesia. In: Gulland FMD, Dierauf LA, Whitman KL, editors. CRC handbook of marine mammal medicine. 3rd ed. Boca Raton (FL): Taylor & Francis Group. p 567–606.
4. McCormick JG, Ridgway SH. 2018. History of the development of anesthesia for the dolphin: a quest to study a brain as large as man’s. Anesthesiol 129(1):11–21.
5. Ridgway SH, McCormick JG. 1971. Anesthesia of the porpoise. In: Textbook of veterinary anesthesia. Baltimore (MD): Williams and Wilkins Co. p 394–403.
6. Bratzke E, Downs JB, Smith RA. 1998. Intermittent CPAP: a new mode of ventilation during general anesthesia. Anesthesiol 89(2):334–40.
7. Bailey J. Innovative Veterinary Medicine Inc.: 101 Marketside AVE, STE 404-402, Ponte Vedra, FL 32081-1541, 2020.