Abstract
During February 1996 and 1997, white-lipped peccary (Tayassu pecari) herds were captured for health evaluations and population dynamics studies at Noel Kempff Mercado National Park, Santa Cruz, Bolivia (Lat. 13°, 35.64′, Lon. 60°, 54.74′), at the base of the northern tip of the Huanchaca escarpment.
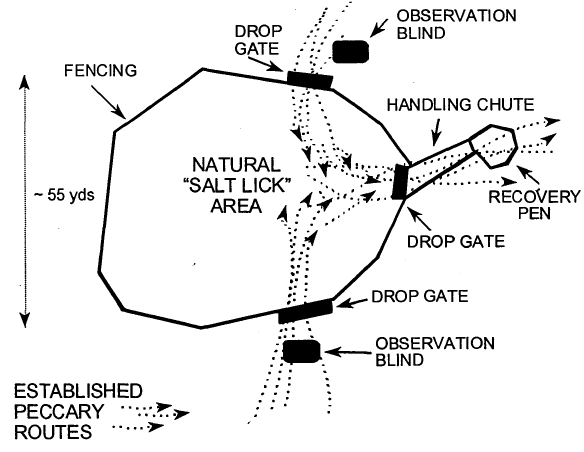
The peccary herds were captured in a large pen built around a frequently used salt lick (salitral) in the forest (Figure 1). Once the peccaries entered the pen, they were darted with a CO2-powered pistol using plastic darts (Telinject, Telinject USA, Saugus, CA). The animals were immobilized using a premixed combination of equal parts tiletamine hydrochloride and zolazepam (Telazol, Fort Dodge, Fort Dodge, IA) using dosages of 80–120 mg for subadults and juveniles and 120–150 mg for adults. Some of the peccaries were supplemented with 100 mg IM doses of ketamine hydrochloride (Ketaset, Fort Dodge, Fort Dodge, IA) when they were not immobilized enough for safe handling. Several animals which recovered slowly from anesthesia were given 2–3 mg of flumazenil (Mazicon, Hoffman-La Roche, Inc., Nutley, NJ) IM to reverse the effects of the zolazepam. Thirty-two adults (>2 years), three sub-adults (2 years) and five juveniles (<2 years) were immobilized, individually identified, and examined. Blood and fecal samples collected for analysis.
Figure 1. Diagram of capture pen used for white-lipped peccaries in Bolivia
Basic hematology was conducted at the field site, including packed cell volumes, total protein, blood smears and WBC counts. Plasma and serum were frozen in liquid nitrogen and later analyzed for serum chemistry, mineral, metal, polychlorinated biphenyl and chlorinated pesticide levels, and infectious diseases.
No evidence of gastrointestinal parasites was found in fecal samples. All peccaries were heavily infested with ticks (Amblyomma sp.). Chlorinated pesticides (aldrin; alpha-BHC; beta-BHC; O,P’-DDD; P,P’-DDD; P,P’-DDE; O,P’-DDT; P,P’-DDT; dieldrin; endrin; heptachlor; heptachlor epoxide; lindane [gamma-BHC]; and nonachlor) and PCBs were not detected in samples analyzed.
Tissues from three peccaries (two adult female and one adult male) that died during anesthesia were evaluated histologically. All three had severe diffuse pulmonary edema and congestion. Two individuals had a multifocal, moderate chronic-active eosinophilic interstitial pneumonia, bronchitis, tracheitis, and pleuritis, and degenerating nematodes were found within sections of the third peccary’s lungs. One peccary had a diffuse severe lymphoplasmacytic epicarditis and multifocal lymphoplasmacytic myocarditis, and another peccary had a chronic-active multifocally extensive severe epicarditis and myocarditis. Two peccaries had diffuse, moderate to severe, chronic-active eosinophilic inflammation in the tunica adventitia of aortic tissue. One of these animals also had a chronic-active eosinophilic moderate diffuse splenic capsulitis. No bacteria or parasites were found associated with these heart lesions.
Two of the peccaries had hepatic capillariasis and all three peccaries had a multifocal, moderate chronic-active and eosinophilic hepatitis. The hepatic capillariasis may have been acquired by the ingestion of tissues from decaying or dead rodents on the forest floor. Two peccaries had a multifocal, moderate chronic-active lymphoplasmacytic interstitial nephritis, and one peccary had a multifocal, moderate lymphoplasmacytic interstitial nephritis.
Despite the negative parasite and ova findings on fecal examination, all peccaries had a diffuse, moderate to severe chronic-active eosinophilic enteritis, suggestive of parasitic inflammation. Two peccaries that had gross evidence of intestinal acanthocephalid also had fibrous and granulomatous nodules within the intestinal wall associated with acanthocephalid attachment. Two peccaries had a multifocal moderate to severe chronic-active eosinophilic dermatitis (most likely associated with cutaneous acariasis found during physical examination).
Infectious disease serology results are listed in Table 1. The negative infectious disease tests indicate that the animals have not been exposed to these pathogens in recent times.
Table 1. Serologic tests performed, the number of positive animals, and the number
of animals tested in the evaluation of infectious disease agent exposure in free-ranging
white-lipped peccaries in Noel Kempff Mercado Nat. Park, Santa Cruz, Bolivia.
|
Agent
|
# Positive (# tested)
|
|
Pseudorabies virus (Aujeszky’s disease)
|
2 (40)
|
|
Porcine parvovirus
|
0 (40)
|
|
Swine influenza virus
|
0 (40)
|
|
Transmissible gastroenteritis
|
0 (40)
|
|
Vesicular stomatitis virus
|
1 (40)
|
|
Anaplasmosis
|
0 (40)
|
|
Bluetongue virus
|
0 (26)
|
|
Johne’s disease (Paratuberculosis)
|
0 (40)
|
|
Foot and mouth disease
|
0 (40)
|
|
African swine fever
|
0 (40)
|
|
Hog cholera
|
0 (40)
|
|
Rinderpest
|
0 (39)
|
|
Brucellosis
|
0 (40)
|
|
Vesicular exanthema of swine
|
24 (40)
|
|
San Miguel sea lion virus
|
22 (40)
|
|
Encephalomyocarditis virus
|
0 (40)
|
|
Leptospirosis (19 serovars)
|
26 (40)
|
|
Hemophilus parasuis
|
0 (17)
|
|
Mycoplasma hyorhinis
|
1(17)
|
|
Streptococcus suis
|
13 (17)
|
Mycoplasma hyorhinis, Hemophilus parasuis, and Streptococcus suis, or other bacterial infections were among the differentials considered for the serosal inflammation seen histologically. Chagas disease and encephalomyocarditis (EMCV) virus were among the differentials considered for the myocardial lesions. However, serologic tests for Chagas disease and EMCV were negative for these individuals. For other herd mates tested, all were seronegative for antibodies to Hemophilus parasuis, and one animal in the herd had a positive Mycoplasma antibody titer. All herd animals tested had Streptococcus suis antibody titers, but these results are equivocal in relation to necropsy findings because most adult domestic swine (Sus scrofa) test positive for this commensal organism.
The high incidence of antibody titers to vesicular exanthema of swine virus and the closely related San Miguel sea lion virus suggests that one or more caliciviruses (which share antigenic qualities) is circulating in the population. Only one out of 14 animals tested in 1996 had positive titers, while 23 out of 26 in 1997 had positive titers. We do not know which strain or strains of calicivirus is responsible for the observed titers nor their effect on the peccary herds studied.
The high incidence of positive leptospirosis titers (65%) shows that the majority of the peccaries have been infected with this organism. The interstitial nephritis seen in the animals that died is consistent with Leptospirosis, and one of the peccaries that died did have a positive leptospirosis titer.
However, immunohistochemistry on renal tissue for leptospirosis was negative in all three animals. Leptospira interrogans can cause illness, reproductive abnormalities and sometimes death in almost all mammal species including humans. Repeated abortions or what appears to be infertility are commonly reported in infected females of other species. Therefore, finding evidence of the presence of this organism has relevance to the population dynamics of Bolivian white-lipped peccaries.
Acknowledgments
We would like to thank the Bolivian national Secretariat for Protected Areas for permission to work in Noel Kempff Mercado National Park, and the National Directorate for the Protection of Biodiversity for help in acquiring the necessary permits. We are extremely grateful to our field assistants Nicolas Tagua and Jose Chuviza whose dedication and initiative were exemplary. Thanks to F.A.N. (Fundación Amigos de la Naturaleza, Santa Cruz, Bolivia) for the logistic support they provided within the park, and to Lidivet for the use of their blood and tissue storage facilities in Santa Cruz. We thank Sue Rosenberg and Marianne Fitzpatrick of the Wildlife Conservation Society (WCS) and Kirk Stuart of the Michigan State Animal Health Diagnostic Laboratory for their help in blood sample analyses. For the identification of parasites, we would like to thank Dr. Eric Hoberg of the U.S. Department of Agriculture Biosystematic Parasitology Laboratory and Dr. Lance Durden of Southern Georgia University. We would also like to thank Drs. Dennis Martin and Nick Karabatsos of the Division of Vector-Borne Infectious Diseases, Centers for Disease Control for the yellow fever testing. Hoffman-La Roche, Inc. kindly provided the Mazicon for use in this study. Much of this study was funded through the Bolivian Sustainable Forestry Project (BOLFOR), which is financed by USAID and the Bolivian Government.