Preliminary Results of Noninvasive Monitoring of the Estrous Cycle in Female Asian Elephants (Elephas maximus) Through Fecal Steroid Analysis
Abstract
For a number of years, estrous cycle monitoring and pregnancy detection in the Asian elephant have been performed using urinary steroid hormone metabolite analysis; this technique presents some practical problems. Monitoring the reproductive status through fecal steroid analysis is possible in this and many other species. The steroid metabolite profiles of female Asian elephants were monitored by enzyme-linked immunosorbent assay (ELISA) to provide detailed information about the estrous cycle and pregnancy in this species and to investigate causes of reproductive failure. Fecal and matched urine samples were noninvasively collected regularly for 6 months from captive female Asian elephants. (n=4 cyclic; n=1 acyclic). The samples were frozen at -20°C. Gas chromatography and mass spectroscopy (GC-MS) procedures were used to investigate the steroid hormone metabolite profile and to identify the major excretory metabolites; no steroid metabolites were found in the concentrated extracted feces of this species using the currently available methodology. The fecal pregnanetriol profile observed in three of the cyclic females showed a clear relation with their matched urinary pregnanetriol profile, and a cyclic pattern was demonstrated. Fecal pregnanetriol values increased from an overall mean of 94.67 ng/g of dry feces (±13.24, range 31.5–219.12 ng/g) during the inter-luteal period to a luteal phase mean of 334.61 ng/g dry feces (±43.48, range 34.35–1035.1 ng/g). All the data collected from the fecal and urinary analysis of pregnanetriol in all five individuals investigated demonstrated a significant relationship between urinary and fecal pregnanetriol. The acyclic individual showed a mean fecal pregnanetriol concentration of 84.91 ng/g (±13.06) and values ranged from 33.17 ng/g to 211.42 ng/g. Fecal steroid hormone metabolite analysis for monitoring estrous cycles in Asian elephants may be used in the future to monitor free-roaming, wild, or semi-wild individuals as well as those in captivity to assist reproductive and conservation programs of this highly endangered species.
Introduction
Assessing the reproductive status of the individuals in captive breeding programs is essential, but invasive techniques are usually not feasible in wild species. Some techniques for noninvasive monitoring of reproductive events in wild animals that have been developed have advantages such as being non-stressful for the individuals that are being monitored, as well as being relatively safe for the human beings involved in the collection of the samples required. They also allow long-term monitoring of species that show long reproductive cycles and require constant sampling and give the opportunity to monitor free-ranging wild animals as well as captive individuals. Most of these techniques are based on the evaluation of hormone levels including metabolites of progesterone and estrogens excreted in urine, feces, and saliva. In the female Asian elephant, the estrous cycle and reproductive events have previously been assessed through the analysis of blood hormone concentrations and more recently by means of urinary steroid hormone metabolite analysis.16,17 Noninvasive monitoring techniques are necessary in this species due to the danger involved in its management in captivity and the impracticability of continuous blood sampling and other invasive procedures in free-ranging individuals.
The Estrous Cycle
Based primarily on measurements of progesterone (and its metabolites) and luteinizing hormone (LH) in plasma and/or urine, the estrous cycle in Asian elephants averages 13–16 weeks.1,12,16,20 Ovulation occurs at the end of each follicular phase.18 It is still uncertain if elephants are normally poly- or mono-ovulatory.13
Metabolism and Excretion of Steroid Hormones
The gonads and the placenta are the main sources of androgens, estrogens, and progestogens. The inter-conversion of steroids may take place peripherally,3 and steroid hormone metabolism occurs in many tissues including the gut, skin, uterus, and mammary gland but mainly in the liver. The biologically inactive metabolites are excreted in the urine, in the bile into feces and to a certain extent in milk and saliva.19 The formation of pregnanetriol follows a separate pathway from that involved in the formation of 20α-hydroxyprogesterone and pregnanediol,15 two of the major progesterone metabolites found in Asian elephant feces according to Hoppen, Diaz de Aguirre, Hagenbeck, Boer and Schwarzenberger.9 Five-beta-pregnanetriol is a major urinary progesterone metabolite in Asian elephant urine.16 Urine is the major route of steroid elimination in most species examined. The route of excretion and the metabolic end products of steroid hormone metabolism can vary considerably between species.19 Fecal and urinary steroid metabolites show similar temporal patterns to plasma concentrations.
Urinary Analysis
Urinary analysis has been used extensively for the detection and quantification of steroids in nondomestic species in different reproductive stages.6,10 Creatinine is usually excreted at a relatively constant rate, and it is used to index the steroid hormone concentrations in each sample in order to adjust variations in urine output.11 Niemuller, et al. identified 5-β-pregnanetriol as the major urinary progesterone metabolite in the Asian elephant.16
Fecal Analysis
Fecal steroid metabolite analyses have been used to assess reproductive status in more than 30 mammalian species.2,6
Methods
Fecal and matched urine samples were noninvasively collected regularly for 6 months from five captive female Asian elephants. According to previous urinary pregnanetriol profiles, four females were known to be cyclic, and one was acyclic. The samples were frozen at -20°C.
GC-MS
GC-MS was performed to investigate the steroid hormone metabolite profile and to identify more accurately the major excretory metabolites, which reflect the reproductive status of the Asian elephant.
Creatinine Determination
After initial thawing, urine samples were analyzed for creatinine concentration by the method of Hodges and Green to correct for variations in fluid intake and urine output.8
Pregnanetriol Enzyme Immunoassay
Immunoreactive 5β-pregnane-3α,17α,20α-triol (pregnanetriol) was measured using a validated modification of the method described by Niemuller, et al.16
20α-Hydroxyprogesterone Enzyme Immunoassay
Fecal 20α-hydroxyprogesterone enzyme immunoassay was performed as described by Hindle, Möstl and Hodges.7
Urinary Analysis
Urinary immunoreactive pregnanetriol was measured using the pregnanetriol enzyme immunoassay described above. Concentrations of urinary pregnanetriol were expressed in ng/mg creatinine. Complete profiles from the five individuals described above were obtained from weekly samples over a period of 6 months.
Fecal Analysis
Sample Preparation and Extraction
Prior to extraction, all fecal samples were thawed and dried in an oven for 18 hours at 60°C. The entire sample was thoroughly mixed, pulverized and the fecal powder was then separated from the hay by sieving through a fine mesh. Five different procedures were used to test which would optimize the extraction of elephant fecal steroids. The extraction procedure which gave the best results was a variation of the extraction procedure described by Möstl, Lehmann and Wenzel: 0.1 g of dried fecal powder was combined with 0.2 g of aluminium oxide, 1.2 ml of methanol and 1 ml of distilled water.14 After being vortexed for 10 minutes, the suspensions were centrifuged for 30 minutes at 2,400 rpm, +4°C and the supernatant was assayed directly after being diluted as appropriate. Aluminium oxide was used to remove visible background pigment.5 This method has proven to be successful for fecal extraction in black rhinoceros for reproductive steroid monitoring.4 Fecal steroid concentrations were expressed in mg/g of dry feces.
Fecal Hormone Assay
The cross-reactivity of fecal extracts was tested with the antisera raised against pregnanetriol and 20α-hydroxyprogesterone, using the enzyme immunoassay methods outlined above. Weekly fecal samples from a complete estrous cycle of one individual were analyzed using both the pregnanetriol and 20α-hydroxyprogesterone assays, and compared with the hormone profile obtained from the analysis of pregnanetriol in matched urine samples. On the basis of the results obtained, all fecal analyses were performed using the pregnanetriol enzyme immunoassay, with samples diluted 1:25 in assay buffer.
Results
GC-MS
From the trials that were carried out, no steroid metabolites were found in the concentrated extracted feces of this species using the currently available methodology.
Fecal 20α-Hydroxyprogesterone Profiles
While capable of discriminating changing concentrations of fecal 20α-hydroxyprogesterone (range 5.8–1054.6 ng/g), the complete profile of one cyclic female did not reveal a cyclic pattern of steroid excretion. No relationship between the fecal profile and the urinary pregnanetriol profile was seen using any of the five extraction procedures.
Fecal Pregnanetriol Profiles
Fecal extracts from all five elephants demonstrated a pattern of pregnanetriol excretion which was temporally correlated with that observed in their matching urinary pregnanetriol profile.
Reproductive Assessment of Individual Asian Elephants
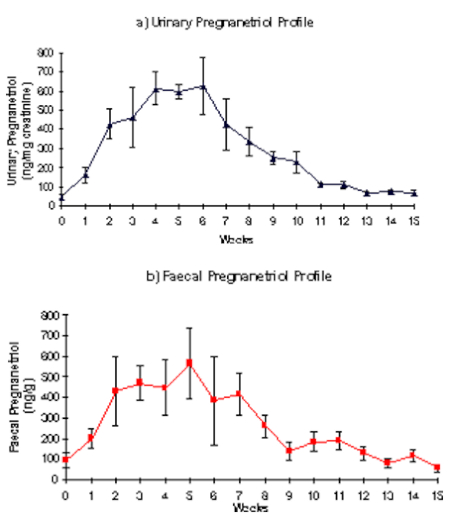
The fecal pregnanetriol profile observed in three of the cyclic females showed a clear relation with their matched urinary pregnanetriol profile and a cyclic pattern was demonstrated. The individual profiles of these three females were used to produce a composite profile of the mean and SEM values of the urinary and fecal immunoreactive pregnanetriol concentrations during one cycle (Figure 1). All data were aligned to week 0, which corresponds to the week before the rise in urinary pregnanetriol (which reflects the start of the luteal phase). Fecal pregnanetriol values increased from an overall mean of 94.67 ng/g of dry feces (±13.24, range 31.5–219.12 ng/g) during the inter-luteal period to a luteal phase mean of 334.61 ng/g dry feces (±43.48, range 34.35–1035.1 ng/g). One of the cyclic females did not show a good relationship between the urinary and the fecal pregnanetriol profile. The acyclic individual showed a mean fecal pregnanetriol concentration of 84.91 ng/g (±13.06) and values ranged from 33.17 ng/g to 211.42 ng/g. No significant difference was found between the mean value of fecal pregnanetriol of an acyclic individual (84.91 ng/g) and the mean value of fecal pregnanetriol (94.67 ng/g) during the inter-luteal phase of the composite profile; however, the acyclic female showed no increase in fecal pregnanetriol excretion indicating the onset of a luteal phase throughout the study period.
Figure 1. Composite profile of the mean±SEM of the (a) urinary and (b) fecal immunoreactive pregnanetriol concentrations during one cycle (n=3)
Discussion
The lack of ability to use the GCMS technique for the elephant could be due to the fact that steroid metabolites are found in relatively low concentrations in their feces. Further studies are required. The fecal 20α-hydroxyprogesterone profile for one of the individuals did not show a cyclic pattern but was capable of discriminating changes in concentration. Comparison of the fecal pregnanetriol profile with the urinary pregnanetriol profile throughout a complete ovarian cycle demonstrated cyclic variations in concentration, and a good correlation between fecal and urine concentrations during all stages of the ovarian cycle. Niemuller, et al. found a significant correlation between immunoreactive pregnanetriol concentrations and plasma progesterone concentrations throughout the ovarian cycle.16 Therefore, we assume that fecal pregnanetriol concentrations are correlated with plasma progesterone concentrations and do reflect the reproductive status of the Asian elephant.
The findings reported in this study and the significant correlation of fecal and urinary pregnanetriol in cyclic and acyclic individuals provide evidence that pregnanetriol is a significant fecal progesterone metabolite in female Asian elephants, and that changes in fecal concentrations can be used to assess the reproductive status of female Asian elephants. The fecal pregnanetriol immunoassay should be further studied in order to evaluate the feasibility of this technique for routine noninvasive monitoring of the reproductive function of captive and wild Asian elephants.
Acknowledgments
The main author wishes to thank The British Council in Mexico City, CONACYT and YUMKA Park, Tabasco, Mexico for their support while working on this project. All data reported on this paper was obtained from the MSc research project of the main author (MSc in Wild Animal Health: Royal Veterinary College–University College of London/The Institute of Zoology-Zoological Society of London, 1998).
Literature Cited
1. Brown, L.J., S.B. Citino, M. Bush, J. Lehnhardt, and L.G. Phillips. 1991. Cyclic patterns of luteinizing hormone, follicle-stimulating hormone, inhibin, and progesterone secretion in the Asian elephant (Elephas maximus). J Zoo Wildl Med 22: 49–57.
2. Brown, J.L., S.K. Wasser, D.E. Wildt, L.H. Graham, and S.L. Monfort. 1997. Faecal steroid analysis for monitoring ovarian and testicular function in diverse wild carnivore, primate and ungulate species. Intl J Mamm Biol 62: 27–31.
3. Cook, B. and G.H. Beastall. 1987. Measurement of steroid hormone concentrations in blood, urine and tissues. In: Steroid Hormones—A Practical Approach. Green, B. and Leake, R.E. (eds.) IRL Press, Oxford. pp. 1–63.
4. Garnier, J., W. Holt, A. Pickard, D. Green, and H. Shaw. 1998. Steroid stability in the faeces of wild black rhinoceros (Diceros bicornis). Proceedings of the Euro-American Mammal Congress. Reig, S. (ed.). Santiago de Compostela. pp. 68.
5. Graham, L.H., J.I. Raeside, K.L. Goodrowe, and R.M. Liptrap. 1993. Measurements of faecal oestradiol and progesterone in non-pregnant and pregnant domestic and exotic cats. J Repro Fertil (Supplement) 47: 119–120.
6. Heistermann, M., E. Möstl, and J.K. Hodges. 1995. Non-invasive endocrine monitoring of female reproductive status: methods and applications to captive breeding and conservation of exotic species. In: Research and Captive Propagation. Ganslosser, U., J.K. Hodges, and W. Kaumanns (eds.) Filander Verlag, Fürth. pp. 36–48.
7. Hindle, J.E., E. Möstl, and J.K. Hodges. 1992. Measurement of urinary oestrogens and 20 alpha hydroxyprogesterone during ovarian cycles of black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses. J Repro Fertil 94: 237–249.
8. Hodges, J.K. and D.I. Green. 1989. A simplified enzyme immunoassay for urinary pregnanediol-3-glucuronide: application to reproductive assessment in exotic species. J Zool 219: 89–99.
9. Hoppen, H.O., L. Diaz de Aguirre, D. Hagenbeck, M. Boer, and F. Schwarzenberger. 1992. Progesterone metabolites in elephant faeces. Proceedings of the First International Symposium on Faecal Steroid Monitoring in Zoo Animals. Schaftenaar, W., Buiter, R.M. and Dielman, S.J. (eds.). Rotterdam. pp. 51–54.
10. Lasley, B.L. and J.F. Kirkpatrick. 1991. Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. J Zoo Wildl Med 22: 23–31.
11. Lasley, B.L. and S.E. Shideler. 1993. Methods for assessing reproduction in nondomestic species. In: Zoo and Wild Animal Medicine 3. Current Therapy. Fowler, M.E. (ed.). W.B. Saunders Co., Philadelphia. pp. 79–86.
12. Mainka, S.A. and Lothrop, C.D. 1990. Reproductive and hormonal changes during the estrous cycle and pregnancy in Asian elephants (Elephas maximus). Zoo Biol 9: 411–419.
13. Mikota, S.K., Sargent, E.L. and Ranglack, G.S. 1994. Medical Management of the Elephant. Indira Publishing House, West Bloomfield. pp. 159–186.
14. Möstl, E., Lehmann, H. and Wenzel, U. 1993. Gestagens in the faeces of mink and cats for monitoring corpus luteum activity. J Repro Fertil (Supplement) 47: 540–541.
15. Niemuller, C.A. 1994. Non-invasive monitoring of reproduction in Asian elephants (Elephas maximus) by urinary endocrine analysis. Ph.D. Thesis, University College London.
16. Niemuller, C.A., Shaw, H.J. and Hodges, J.K. 1993. Noninvasive monitoring of ovarian function in Asian elephants (Elephas maximus) by measurement of urinary 5-beta-pregnanetriol. J Repro Fertil 99: 617–625.
17. Plouzeau, E., Da Cunha, S. and Shaw, H.J. 1994. The estrous cycle of the Asian and African elephants, Elephas maximus and Loxodonta africana: techniques to monitor reproduction of females in captivity. Revue de Médecine Vétérinaire 145: 905–911.
18. Schmidt, M.J. 1993. Breeding elephants in captivity. In: Zoo and Wild Animal Medicine 3. Current Therapy. Fowler, M.E. (ed.). W.B. Saunders Co., Philadelphia. pp. 445–448.
19. Schwarzenberger, F., Palme, R., Bamberg, E. and Möstl, E. 1997. A review of faecal progesterone metabolite analysis for non-invasive monitoring of reproductive function in mammals. Intl J Mamm Biol 62: 214–221.
20. Taya, K., Komura, H., Kondoh, M., Ogawa, Y., Nakada, K., Watanabe, G., Sasamoto, S., Tanabe, K., Saito, K., Tajima, H. and Narushima, E. 1991. Concentrations of progesterone, testosterone and estradiol-17 beta in the serum during the estrous cycle of Asian elephants (Elephas maximus). Zoo Biol 10: 299–307.