Department of Veterinary Physiological Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) such as flunixin meglumine (FLX) and ketoprofen (KET) have been used in both small and large animals as anti-inflammatories and analgesics. Plasma levels of NSAIDs do not reflect physiologic or pharmacologic activity. Thromboxane (TBX), an inflammatory mediator whose production is inhibited by NSAIDs, plasma measurements can be used to predict length of drug action. Plasma TBX levels were measured following IM administration of 5 mg/kg KET or FLX to mallard ducks (Anas platyrhynchos). Compared to baseline, TBX was suppressed significantly for 4 h for both drugs. Decline of plasma TBX levels, over 48 h in the control group, may indicate that stress plays a role in TBX suppression. The NSAIDs, FLX and KET appear to suppress the inflammatory process but cannot be directly correlated to analgesia.
Introduction
Flunixin meglumine (FLX) and ketoprofen (KET) are potent non-steroidal anti-inflammatory drugs (NSAIDs) used therapeutically in human and veterinary medicine to alleviate pain and decrease inflammation.5,9,10 In avian species, FLX has been recommended for pain relief,11 but use of KET has not been reported. The pharmacokinetics and pharmacodynamics of these NSAIDs in birds are unknown. In dogs and cats, both FLX and KET provide appropriate analgesia for surgical pain with a half-life of 3–4 h and 2–3 h, respectively.9 Anti-inflammatory, analgesic, antipyretic, and antithrombotic actions of NSAIDs are brought about by inhibition of prostaglandin production. More specifically, NSAIDs block the access of arachidonic acid to its binding site on the cyclo-oxygenase enzyme, preventing its conversion to prostaglandin and thromboxane B2 (TBX).5 NSAIDs accumulate at sites of inflammation as they are weak acids10 and possibly because of increases in vascular permeability, blood flow, and protein passage to these sites4. Plasma levels of NSAIDs do not reflect physiologic or pharmacologic activity; therefore, plasma TBX levels are more accurate indicators of length of drug action.5,13 The purpose of this study was to investigate the pharmacodynamics of the two NSAIDs, FLX and KET, in mallard ducks (Anas platyrhynchos) by measuring plasma TBX levels.
Materials and Methods
Sixteen captive raised adult mallard ducks were used for the study. The ducks were of equal sex ratio (eight male, eight female), with average body wt of 1055±114 g and 943±85 g, respectively. Ducks, in an equal sex ratio, were randomly assigned to three treatment groups: (1) control (n=4), (2) flunixin meglumine (FLX, 5 mg/kg, n=6, Banamine, Schering-Plough Animal Health, Point-Claire, QC, Canada) or (3) ketoprofen (KET, 5 mg/kg, n=6, Anafen, Rhône Mérieux Inc. Athens, GA). Two ducks in the control group had a jugular catheter placed during isoflurane (IsoFlo, Abbot Laboratories Limited, Saint-Laurent, QC, Canada) anesthesia and bupivacaine (2 mg/kg, Marcaine, Sanofi Winthrop, Chatham, ON, Canada ) local anesthetic, and two ducks were anesthetized but received neither a jugular catheter nor bupivacaine. Ducks in the latter group had blood samples taken from either the jugular or brachial veins. All ducks in the FLX and KET groups had a jugular catheter placed during isoflurane anesthesia, but bupivacaine was not given. During isoflurane anesthesia, the right jugular vein was isolated surgically and a 20-ga, 5.0-cm catheter was inserted for the purpose of repeated blood sampling.
Blood samples of 0.3 ml were drawn into heparinized syringes at -1 h (60 min prior to catheter placement) and 0 h (after isoflurane induction) from the brachial vein. Immediately after induction, either FLX or KET was administered IM into the left pectoral muscle. Equal volumes (5.1 ml) and concentrations of NSAID were given by diluting with sterile saline. Ducks in the control group received an equal volume of saline IM in the left pectoral muscle. Blood samples of 0.5 ml were taken at 15 min, 30 min, and 1, 2, 4, 6, 12, 24, 36, and 48 h. Time 0 and 15 min samples were taken during anesthesia. Blood samples were placed in integrated plasma separation tubes with lithium heparin (Sherwood Davis and Geck Medical, St Louis, MO, USA). Samples were centrifuged and frozen at -20°C until analysis were performed. Duplicate samples from each bird were analyzed using a Thromboxane B2 Enzyme Immunoassay Kit (Cayman Chemicals, AR, USA). The coefficient of variation for this study was 5.44±4.71 (x±SD). Feces were tested at 0, 24, and 48 h for the presence of hemoglobin using Haematest tablets (Miles Canada Inc., Etobicoke, ON, Canada) to evaluate for NSAID-associated gastrointestinal bleeding. All ducks were maintained individually in cages (75 cm3) with a 20x40 cm pool and fed duck and goose grower ad libitum. Ducks were euthanatized for complete necropsy at the end of the study. Histologic analysis of muscle, heart, kidney, liver, spleen, and gastrointestinal tract are pending.
Data are reported as mean±SEM. Wilcoxon signed-rank tests were used to determine if there were significant differences between time -1 and 0 h before combining results. Plasma TBX data were analyzed using a repeated measures analysis of variance (ANOVA) for the average value derived from duplicate samples where the same individuals were sampled at each time point. Data were log transformed to avoid violating the normality assumption of the ANOVA. Where significant differences occurred, a multiple comparison using a contrast statement for repeated measures was used to compare results with baseline (average of time 0 and -1 h). Results were considered significant when p<0.05.
Results
Results from one male duck in the FLX group were excluded when necropsy and histologic findings consistent with systemic mycobacterial disease were found. As there was no difference in TBX suppression between males and females or between control groups, results from both sexes and control groups were combined. TBX was suppressed in all birds following administration of either FLX or KET (Figures 1 and 2). Maximal TBX suppression occurred at 30 min and was still significantly suppressed at 4 h (F=30.3, df=10, 90, p=0.0001, Figure 1). There was no significant difference between drugs (F=1.05, df=10,90, p=0.392) but TBX suppression by KET did not appear to be as long lasting as with FLX (Figure 2).
Figure 1. Comparison of mean (SEM) plasma thromboxane (TBX, [pg/ml]) in mallard ducks following IM injection of flunixin meglumine (FLX, [5 mg/kg]), ketoprofen (KET, [5 mg/kg]), and saline controls
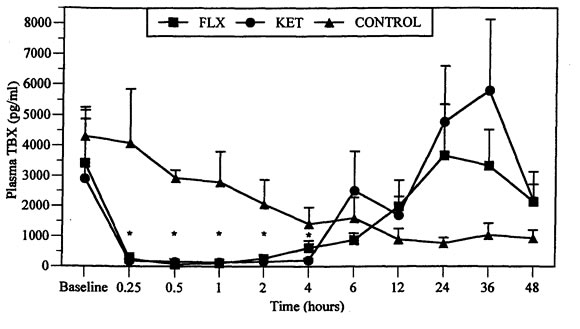
*Significantly different from baseline values, p<0.05.
Figure 2. Percent thromboxane (TBX) suppression compared to baseline in mallard ducks following IM injection of flunixin meglumine (FLX, [5 mg/kg]) and ketoprofen (KET, [5 mg/kg])
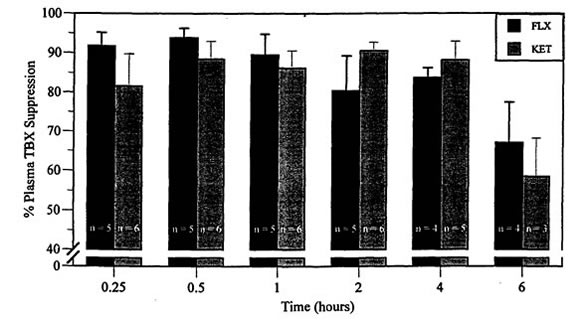
n=5 for FLX and 6 for KET at 0.25, 0.5, 1, and 2 h.
n=4 for FLX and 5 for KET at 4 h.
n=4 for FLX and 3 for KET at 6 h.
Ducks in the control group had a gradual decline in TBX from the start of the experiment (time -1 and 0 h) to 48 h. The decline was not related to any obvious circadian rhythm, the highest mean values (4299±958 pg/ml) were recorded at baseline (times -1 h and 0 h) and the lowest value (361±180 pg/ml) occurred at 24 h. At 36 (787±394 pg/ml) and 48 h (530±265 pg/ml), TBX values were marginally higher than at 24 h but not as high as at baseline (Figure 1).
At necropsy, all birds given FLX had severe, focally extensive muscle necrosis of approximately 1–2 cm3 at the injection site. No other gross abnormalities were found on postmortem and histology results are pending. None of the ducks had evidence of gastrointestinal bleeding.
Discussion
In this study, plasma TBX was significantly suppressed by both drugs for at least 4 h. Similar studies of these two NSAIDs have shown that TBX suppression in mammals is longer. Significant suppression may be short as 4 h or as long as 24 h, with levels returning to normal by 12 h7,12 or 48 h, respectively.7
Compared to baseline, maximum TBX suppression was achieved by 30 min, which may indicate that uptake and distribution is more rapid in mallard ducks than reported in mammalian species. Significant suppression (73 to 96%) was maintained for only 4 h. The short inhibition of TBX may be related to the higher metabolic rate of birds. Nonpasserine birds have a minimal energy requirement that averages almost 10% more than that of placental animals.11 The increase in TBX production seen after suppression in this study is similar to that reported in other following studies but the cause is unknown.3,8
Return of TBX to baseline values was difficult to estimate in this study. The gradual decline in control TBX concentration over 48 h, as seen in the control ducks, has not been reported in similar studies. The stress of being confined, isolated, and multiple blood sampling may have resulted in high levels of circulating corticosterone, which, through lipocortin production, indirectly can limit the activation of the inflammatory cascade.1,2
It is unknown if the degree of TBX inhibition can be correlated with the degree of analgesia provided by the NSAID. A possible mechanism for the peripheral analgesic effects of NSAIDs is through suppression of the cyclo-oxygenase pathway.4,5 As FLX and KET appear to cause significant suppression of TBX by 30 min and continue for at least 4 h, they may be useful during the surgical procedures and may also reduce altered behavior postoperatively. Further studies are required to determine the effectiveness of preemptive NSAID administration as analgesics in birds.
Muscle necrosis seen in the ducks, associated with FLX injections, has been observed in northern bobwhite. However, a dose of 32.0 mg/kg/day in quail over the course of 7 days was necessary to induce the lesions.6 Mallard ducks may be more susceptible to these detrimental effects of FLX but further studies are required to establish their susceptibility to lesions.
Flunixin meglumine and KET can produce significant inhibition of TBX and therefore the inflammatory cascade for at least 4 h. Administration of KET may benefit waterfowl research if it can provide postoperative analgesia and thus reduce altered behavior and weight loss in birds after surgical implantation of radiotransmitters. Flunixin meglumine may not be a suitable NSAID for use in ducks because of the muscle necrosis produced at the injection site. More research is needed to determine if preemptive administration of NSAIDs can reduce deleterious effects of postoperative pain.
Acknowledgments
This research was supported by the Canadian Wildlife Service, Delta Waterfowl Foundation, Ducks Unlimited’s Institute for Wetland and Waterfowl Research, Interprovincial Undergraduate Student Summer Research Award, and the Wildlife Health Fund, University of Saskatchewan. We thank Susan Cook for assistance in sample analysis and Dr. Robert Brua for aid in statistical analysis and writing of this abstract.
Literature Cited
1. Blackwell, G.I., R. Carnuccio, M. DiRosa, et al. 1980. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 287:147–149.
2. Breazile, J.E. 1987. Physiological basis and consequences of distress in animals. J Am Vet Med Assoc. 191:1212–1215.
3. Hardee, M.M. and J.N. Moore. 1986. Effects of flunixin meglumine, phenylbutazone and a selective thromboxane synthetase inhibitor (UK-38,485) on thromboxane and prostacyclin production in healthy horses. Res Vet Sci. 40:152–156.
4. Higgins, A.J. and P. Lees. 1984. The acute inflammatory process, arachidonic acid metabolism and the mode of action of anti-inflammatory drugs. Equine Vet J. 16:163–175.
5. Kantor, T.G. 1986. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. 6:93–103.
6. Klein, P.N., K. Charmatz, and J. Langenberg. 1994. The effect of flunixin meglumine (Banamine) on the renal function in northern bobwhite (Colinus virginianus): an avian model. Proc Am Assoc Zoo Vets, pp. 128–131.
7. Landoni, M.F. and P. Lees. 1995. Comparison of the anti-inflammatory actions of flunixin and ketoprofen in horses applying PK/PD modelling. Equine Vet J. 27:247–256.
8. Lees P. and A.J. Higgins. 1985. Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs I: the horse. Equine Vet J. 17:83–96.
9. Mathews, K.A. 1996. Nonsteroidal anti-inflammatory analgesics to manage acute pain in dogs and cats. Comp Cont Ed. 18:1117–1123.
10. Mortensen, M.E. and R.M. Rennebohm. 1989. Clinical pharmacology and use of nonsteroidal ant-inflammatory drugs. Pediatr Clin North Am. 36:113–1138.
11. Ritchie B.W. and G.J. Harrison. 1994. Formulary. In: Avian Medicine: Principles and Application. Ritchie B.W., G.J. Harrison and L.R. Harrison (eds.). Wingers Publishing Inc. Florida. Pp. 457–478.
12. Semrad, S.D., R.A. Sams, and S.M. Ashcraft. 1993. Pharmacokinetics of and serum thromboxane suppression by flunixin meglumine in healthy foals during the first month of life. Am J Vet Res. 54:2083–2087.
13. Soma, L.R., C.E. Uboh, J. Rudy, and J. Fegely. 1992. Plasma concentrations of flunixin in the horse: its relationship to thromboxane B2 production. J Vet Pharmacol Therap. 15:292–300.