Abstract
Vitamin E deficiency in piscivorous birds was recognized in zoos during the early 1980s as a myodegenerative process.1,3,5,6 From these case studies, vitamin E supplementation was recommended for fish-eating birds consuming frozen, thawed fish. Excessive vitamin E supplementation was documented in the late 1980s as a vitamin K-responsive coagulopathy causing death in a pelican species.7
The Dallas Zoo has displayed African pelican species since the 1980s. Vitamin E supplementation was provided on a daily basis, although by different formulations. Since 1996, Sea Tabs (vitamin E 50 IU/tablet, Pacific Research Laboratories, Inc., El Cajon, CA, USA) have been utilized. The pelican flock was considered appropriately supplemented for vitamin E, based on 100 IU vitamin E/kg of fish fed.2,3 However, between November 2001 and January 2002, three of three East African white pelicans (Pelecanus onocrotalus) and two of three pink-backed pelicans (Pelecanus rufescens) died due to concurrent rhabdomyolysis, steatitis, and coagulopathy (Table 1).
Table 1. Histories and observations on five pelican deaths
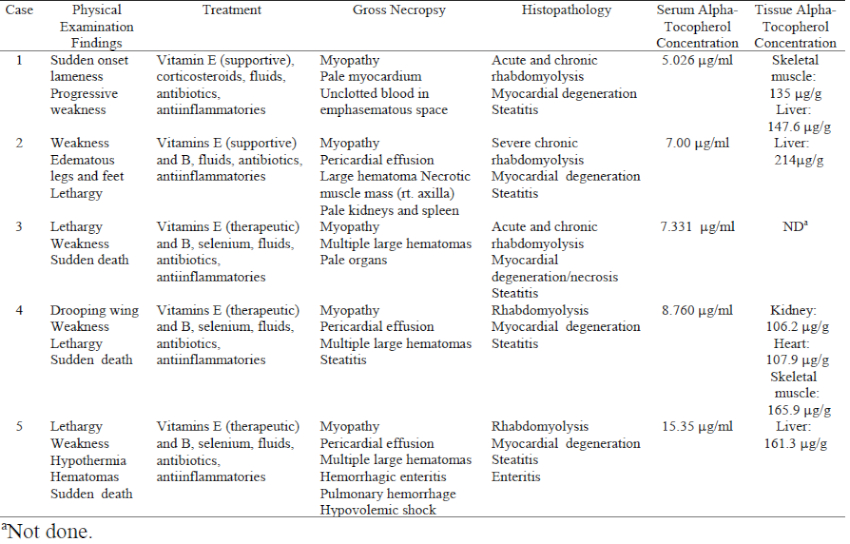
The pelicans were housed in a natural substrate and artificial river exhibit for at least 7 years. The only bird to survive was a pink-backed pelican acquired 1 year prior to the fatalities. These birds had been fed frozen/thawed trout, capelin, and smelt until late 2000, when the diet was changed to 1.5 kg of frozen/thawed smelt and capelin daily per bird with the vitamin E supplement adjusted accordingly. Substantial exhibit renovation and relocation of birds within the exhibit began in September of 2001. In early November, a male East African white pelican (case 1) presented with acute onset pelvic limb lameness and proprioceptive deficits but had no palpable or radiographic abnormalities, and no history of trauma. Supportive treatment was initiated consisting of crystalloid fluids (10 ml/kg SQ SID, Normosol, Abbott Laboratories, North Chicago, IL, USA), vitamin E/selenium (1 ml/kg IM SID, Bo-SE, Schering-Plough Animal Health, Union, NJ, USA, ) and methylprednisolone (15 mg/kg IM SID, Solu-Delta-Cortef, Pharmacia & Upjohn, Kalamazoo, MI, USA) for the first three days after presentation, followed by trimethoprim sulfa (30mg/kg SQ SID, sulfadiazine/trimethoprim, Schering Plough Animal Health Corp.) for ten days. When no response was noted, fluids were extended and anti-inflammatories (1 mg/kg IM EOD, Banamine, flunixin meglumine, Schering-Plough Animal Health Corp.), and repeated vitamin E/selenium (1 ml/kg IM SID for 5 days) were initiated during the 10–20 days after presentation. The bird died 20 days after presentation without substantial improvement.
Within 1 week of this fatality, a female pink-backed pelican (case 2) presented with profound lethargy, pitting edema of the legs, and inability to stand. Supportive treatment as above was provided as well as DMSO gel (topically to podotheca, SID for 2 days, Fort Dodge Animal Health, Fort Dodge, IA, USA), and within 48 hours ambulation returned and the lethargy and edema resolved. Once stabilized, the pelican received a more critical diagnostic evaluation due to the recent flock mate death. The only substantial clinical pathology changes were elevated creatinine phosphokinase (CPK) and a heterophilic leukocytosis. Radiographs revealed obvious renal silhouettes, undifferentiated abdominal portion of the coelomic cavity, and a soft tissue mass in the right axilla. A standard intracoelomic laparoscopy performed the following day revealed pale lateral body wall musculature, pale kidneys, and ample fat reserves. Incisional biopsy of the axillary mass was grossly consistent with a hematoma and large pieces of necrotic muscle. After 30 minutes of slow but gradual recovery from anesthesia, the bird was found dead.
The remaining four pelicans were promptly moved to indoor holding for physical examination and diagnostic sample collection. A symptomatic parenteral regimen of fluids, vitamin E/selenium, and flunixin meglumine was initiated using the previously described dosages and frequency of administration. Serial blood samples on all birds revealed progressively elevated CPK and a leukocytosis with a mature heterophilia. From late December 2001 to mid-January 2002, two East African white pelicans (cases 3 and 4) and one pink-backed pelican (case 5) presented for lethargy and weakness, each dying within 10 minutes of presentation.
Consistent gross necropsy findings on all birds revealed pallor and streaking of the pectoral and thigh musculature, cardiac pathology (either hydropericardium or myocardial streaking), subcutaneous hemorrhage without indication of trauma, and gelatinous or necrotic adipose tissue. Histopathology confirmed severe chronic-active rhabdomyolysis, steatitis, and myocardial degeneration with necrosis in all birds.
Muscle lesions present in all the pelicans are consistent with clinical hypovitaminosis E. However, the alpha-tocopherol serum concentrations of all birds were within expected ranges for avian piscivores, and alpha-tocopherol tissue concentrations were within normal limits for carnivores in the liver of cases 1 and 2 and skeletal muscle of case 1.2,3 Alpha-tocopherol is the more active form of vitamin E, which is an antioxidant that protects cell membranes from products of metabolism and lipid oxidation by scavenging free radicals.3 It is an integral nutrient in animals with diets high in polyunsaturated fatty acids, which are found in high concentrations in marine fish.3,5 Captive piscivores are often fed frozen-thawed fish. This practice produces a potential for polyunsaturated fatty acids to convert to free radicals, thus creating a necessity for vitamin E supplementation.3
Studies also indicate that vitamin E levels in plasma are markedly decreased by excessive vitamin A supplementation, due to vitamin A antagonizing vitamin E absorption from the gastrointestinal tract.2,4 These pelicans were receiving multivitamin supplements containing vitamin A (1,000 IU per tablet), in addition to that of the fish in the diet. However, the serum and tissue concentrations of alpha tocopherol were within normal limits, indicating that absorption of the vitamin was not impeded. In fact, vitamin E tissue concentrations were approaching higher ends of the normal ranges. Although the exact mechanism is unknown, oversupplementation with vitamin E can produce a vitamin K-responsive coagulopathy.7 It is believed that metabolites of vitamin E inhibit the function of vitamin K. These metabolites of vitamin E are the suggested source of hemorrhage and hematomas noted clinically in these birds, rather than the exudative diathesis of transparent fluid and slight hemorrhage characterized by hypovitaminosis E.3
The literature has extensive documentation of rhabdomyolysis due to hypovitaminosis E, as well as individual cases of coagulopathy due to hypervitaminosis E. However, the combination of both conditions has not yet been documented in avian piscivores in the face of daily vitamin E supplementation. Potential sources of this “vitaminopathy” include: a fat-soluble vitamin imbalance due to the amount of vitamin A in the supplement coupled with that in the fish fed, the interaction between the fat-soluble vitamins in the gastrointestinal tract with over- or under-supplementation of individual vitamins, the quality and quantity of fish being fed, and the amount of stress incurred by these animals during the months prior to presentation. Another consideration is inappropriate supplement availability from a synthetic vitamin E source versus a natural vitamin E that would have higher bioavailability and effectiveness at the cellular level. It is proposed by this series of cases that the pelicans had endured acute onset, but protracted stress, possibly coupled with poor-quality fish, which increased the need for vitamin E. Analysis of fish fed indicated expected amounts of vitamin A and vitamin E, leaving increased utilization as the most likely explanation for rhabdomyolysis. Normal serum and tissue concentrations analyzed postmortem indicated that the vitamin E was absorbed and should have been available. However, the tissues were damaged and unable to utilize the antioxidant or to heal. From this experience, a recommendation is to supplement pelican species with a vitamin E source, preferably natural, at 100 IU/kg fresh-weight fish and decrease environmental stressors. In times of apparent stress, periodic quantitative analysis of the diet fish, as well as monitoring of serum alpha-tocopherol in the birds, should be initiated to adjust supplementation to meet the increased need.
Literature Cited
1. Campbell, G., and R.J. Montali. 1980. Myodegeneration in captive brown pelicans attributed to vitamin E deficiency. J. Zoo Wildl. Med. 11: 35–40.
2. Crissey, S.D, P.M. McGill, and A. Simeone. 1998. Influence of dietary vitamins A and E on serum “α and (γ tocopherols, retinal, retinyl palmitate and carotenoid concentrations in Humboldt penguins. Comp. Biochem. Physiol. 121A: 333–339.
3. Dierenfeld, E.S. 1989. Vitamin E deficiency in zoo reptiles, birds, and ungulates. J. Zoo Wildl. Med. 20: 3–11.
4. Frigg, M., and J. Broz. 1984. Relationships between vitamin A and vitamin E in the chick. Internat. J. Vit. Nutr. Res. 54: 125–134.
5. Geraci, J.R., and D.J. St. Aubin. 1980. Nutritional disorders of captive fish-eating animals. In: Montali, R.J., and G. Migaki (eds.). Comparative Pathology of Zoo Animals. Smithsonian Institution, Washington, D.C. Pp. 41–49.
6. Nichols, D.K., and R.J. Montali. 1987. Vitamin E deficiency in captive and wild piscivorous birds. Proc. First Int. Conf. Zool. Avian Med., Turtle Bay, Hawaii. Pp 419–421.
7. Nichols, D.K., M.J. Wolff, L.G. Phillips, and R.J. Montali. 1989. Coagulopathy in pink-backed pelicans (Pelicanus rufescens) associated with hypervitaminosis E. J. Zoo Wildl. Med. 20: 57–61.