Morbidity and Mortality Related to Hypoglycemia and Chronic Energy Malnutrition in Captive Giraffe
Abstract
Peracute mortality syndrome (PMS) is a well-described syndrome of captive giraffe.8,11 Several causes of peracute mortality have been looked at and excluded from consideration as the causative agent or process.8 It has been suggested that an underlying energy deficiency may have an important role or perhaps be the predisposing cause of the problem. It is proposed that hypoglycemia is the immediate cause of PMS and that a chronic energy malnutrition may be a prerequisite for this syndrome. Chronic energy malnutrition may also be responsible for other health issues seen in captive giraffe.
Case Reports
Case 1
An 8-yr-old hand-reared, gravid reticulated giraffe (Giraffa camelopardalis reticulata) collapsed and died during a routine shifting procedure at Busch Gardens Tampa (BGT). The animal had been under observation due to impending parturition and placed in a barn due to diarrhea and lethargy 14 days prior to death. At that time (day 0) the animal was anemic (PCV 20%), with a leucocytosis (22,000/µl) with a neutrophilia, occult positive stool, and an egg count of 4,000 eggs/g (epg) feces. Activated partial thromboplastin time (aPPT) and thrombin clot time were prolonged compared to in-house normal. The giraffe’s body condition was judged to be poor. Treatment at the time consisted of injectable levamisole, oral electrolyte solutions, and an oral vitamin and mineral supplement. Urinalysis was unremarkable but feces were still occult positive on day 5. Blood sampling on day 6 reveal persistent leucocytosis (19,500/µl), elevated CPK (5688 IU/L), and hypoglycemia (47 mg/dl). The fecal egg count had dropped to 1,800 epg and the feces were still occult positive. On day 7 the dam was given 25 ml of B-complex injected intramuscularly. Serum progesterone was at 5 ng/ml and fetal viability was questioned. Complete blood count (CBC) on day 8 revealed the leucocytosis seen before but toxic neutrophils were noted this time. Packed cell volume was 21% and blood glucose was 55 mg/dl. The stools had firmed up considerably. Five liters of lactated Ringer’s solution were given subcutaneously. Fluids were repeated on day 9 and 12.14 g of florfenicol were given subcutaneously. A rectal exam was performed the next day and the fetus determined to be viable. The giraffe did not eat well for the next two days and was witnessed to collapse and die. A fully developed but extremely small, 55-lb female calf, was removed via flank incision. The calf only survived for 30 min. Blood samples of the dam, taken while the calf was being removed, revealed blood glucose of 20 mg/dl. Gross findings revealed a lack of body fat and serous atrophy of pericardial fat. Histopathologic findings included serous atrophy of mesenteric and epicardial fat, lymphohistiocytic rumenitis, pulmonary congestion, and mild membranoproliferative glomerulonephropathy.
Case 2
A 6.5-yr-old hand-reared, gravid reticulated giraffe collapsed and died after a 10-day episode of partial anorexia believed to involve an infected tooth. This giraffe had been isolated from the herd for 13 mo due to a single fecal culture positive for Mycobacterium paratuberculosis. Intensive screening for the isolation period revealed no further positive cultures and the giraffe was released back to the herd. The animal was eating only browse and hay while leaving all pelleted ration. The giraffe had a poor body condition, given a score of 3/8.12 Blood was collected on day 4 and revealed a leucocytosis (19,700/µl), with a neutrophilia, elevated CPK (1937 IU/L), and hypoglycemia (72 mg/dl). Bloodwork was repeated two days later with the WBC within normal limits, CPK climbing (2181 IU/L), elevated creatinine (2.5mg/dl), and more severe hypoglycemia (47 mg/dl). The giraffe was found dead in the barn on day 10. Videotape overnight showed the dam to simply collapse at 2:45 a.m. without any evidence of a struggle. Blood collected 5 hr after death had serum glucose of 3 mg/dl. Necropsy revealed serous atrophy of fat stores in the mesenteric, perirenal, and epicardial fat stores. The pancreas was atrophied. The last right maxillary molar was slightly loose but not believed to be significant. Generalized muscle atrophy was also noted. A 73-lb male calf was in a normal presentation with the forefeet at the internal os of the cervix. Histopathologic findings also included serous atrophy of mesenteric and epicardial fat, lymphohistiocytic rumenitis and pulmonary congestion.
Discussion
Both of the preceding cases are classic examples of PMS: an underlying stressor, sudden death, and post-mortem findings consistent with the case definition of PMS. Based on the blood glucose levels preceding death and those taken shortly after death, hypoglycemia is believed to be the immediate cause of death. By comparison, serum collected on other ungulate species taken from animals found dead in the morning all had blood glucose levels of greater than 40 mg/dl (n=4). The circumstances of the deaths and the fact that they were both witnessed help rule out other causes of sudden death.
Hypoglycemia and energy deficient states have been mentioned as possible causes or underlying factors in PMS since the condition was classified8,11 but only recently have been given serious consideration. Table 1 lists the possible causes of peracute death proposed in Fowler’s original description. Most of these causes can be excluded by gross and histologic findings along with history. Cholinergic bradycardia and hypovolemia will be difficult to prove and may be excluded if other causes are more easily explained. Increased vagal tone is a common cause of cholinergic bradycardia and although toxins10 and drugs can also induce cholinergic bradycardia, these have not been implicated in cases of PMS. Gastrointestinal inflammation can elicit a vagal induced bradycardia4 but will be difficult to determine in giraffe. Exocrine pancreatic insufficiency like syndrome (EPI) has been reported13 and pancreatic atrophy is an extremely common finding in PMS.
Table 1. Possible causes of peracute mortality8
- Adrenal exhaustion
- Anaphylaxis
- Brain contusion
- Cerebral vascular accident
- Cholinergic bradycardia
- Congestive heart failure
- Coronary arterial occlusion
- Endotoxic shock
- Gastric dilatation
- Gunshot
- Hemorrhage
- Hypocalcemia
- Hypoinsulinemia
- Hypoglycemia
- Hypovolemia
- Poisoning
- Pulmonary edema
- Pulmonary embolism
- Suffocation
- Ventricular fibrillation
Pancreatic hypoplasia and EPI are not as likely to interfere with the endocrine function of the pancreas. Pancreatitis affects exocrine and endocrine function, but pancreatic atrophy is the pathology associated with PMS and therefore, hypoinsulinemia seems less likely. Altered calcium to phosphorus ratio has been noted previously1,7 and was seen in the cases above. In the second case however, the hypocalcemia was reversed when the giraffe stopped eating concentrates and consumed only browse and hay. A closer look at ionized calcium is warranted.
Hypoglycemia certainly had a major role in the deaths of the cases above. Ante mortem findings suggested cachexia and worsening hypoglycemia. A stressful event, parturition, expended all remaining reserves and the giraffe simply collapsed. Several other giraffe at BGT have had similar episodes with other stressors but not had the deaths witnessed. One ante-mortem finding they do have in common is the chronic cachexia. The lack of a cold stressor may allow giraffes in warmer climes to catabolize muscle to maintain blood glucose. Cold stress and hypothermia induces a hyperglycemic response and animals without reserves may succumb to acute hypoglycemic episodes. Ruminants in general are primarily gluconeogenic with very limited capacity for glycolysis and this may explain why animals in apparently good condition may suffer from cold stress induced PMS. Glucose levels in captive giraffe are actually reported to be higher than in their wild counterparts2 and elevated blood glucose is a well-documented indicator of stress. Sub-optimal nutrition may in fact not only provide an inappropriate energy source but also may induce a chronic inflammatory condition of the gastrointestinal tract (Figure 1) and become a chronic stressor in itself.
Figure 1. Proposed pathways involving feeding, energy status, and clinical entities in captive giraffe
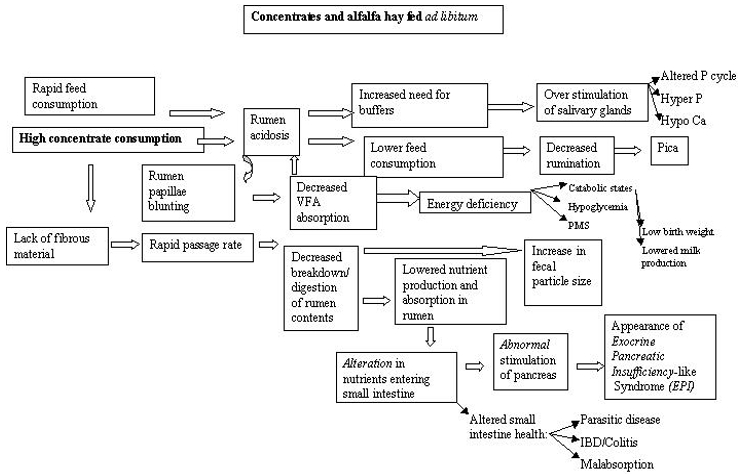
Low birth weights, maternal rejection, and failure of passive transfer may all be directly or indirectly related to chronic energy malnutrition. Birth weights of wild giraffe are reported to be approximately 70 kg.3 Captive born giraffe are reported to have a range of normal or expected birth weights of between 50–60 kg.9 At BGT there appears to be a strong association between low birth weights (<60 kg), hand rearing and mortalities with the lowest birth weights typically having the highest risk of mortality. Low birth weight and mortality have been reported elsewhere7 and seem to be common anecdotally. Energy status of the dam influences birth weight and small, weak calves frequently require hand rearing and are more susceptible to infections than heavier calves. Maternal rejection and failure of passive transfer may both be related to lactation status of the dam.
Marginal or negative energy balance in the dam leads to poor milk production and medical records at BGT indicate that even dams with calves with adequate birth weights will still have calves removed for hand-rearing due to lack of udder development and maternal neglect. Several facilities have encountered this and at times overcome the problem by sedating the dam with xylazine.6 Other noted causes include mastitis but supporting evidence is not strong.7 In these cases, xylazine may have produced an analgesic or sedative effect for the dam but is also known to induce hyperglycemia in many domestic species with profound effects on cattle.5 If indeed the xylazine induced hyperglycemia facilitated milk production, it did so in the face of hypoinsulinemia. Given the success of the reported case and others, it would appear that hypoinsulinemia does not play a major role in PMS. Failure of passive transfer in giraffe calves14 may also result from inadequate milk production and rejection by the dam. It is difficult to evaluate globulin levels in the dam’s colostrum and the failure may simply be one of underfeeding due to weak small calves or poor production from a marginally healthy dam.
Literature Cited
1. Ball RL. 2000. Hematology, serum chemistry, blood coagulation profiles and serology of physically restrained captive reticulated giraffe (Giraffa camelopardalis) and the American Association of Wildlife Veterinarians Joint Conference.
2. Clauss M. 1998. Feeding Giraffe (Giraffa camelopardalis) [masters thesis]. University of London.
3. Dagg A. 1976. The Giraffe, Its Biology, Behaviour and Ecology. Van Nostrand Reinhold, New York.
4. De Giorgio R, B. 2001. Intestinal inflammation and activation of sensory nerve pathways: a functional and morphological study in the nematode infected rat. Gut. 49:822–7.
5. Eichner RD, RL Prior, WG Kvasnicka. 1979. Xylazine-induced hyperglycemia in beef cattle. American Journal of Veterinary Research. 40:127–129.
6. Fischer MT, RE Miller, EW Houston. 1997. Serial tranquilization of a reticulated giraffe (Giraffa camelopardalis reticulata) using xylazine. J Zoo Wild Med. 28:182–4.
7. Flach EJ. Chronic loss of condition with persistent neutrophilia in a reticulated giraffe (Giraffa camelopardalis). Proceedings of the Spring Meeting of the British Veterinary Zoological Society. pp.33–36. 1997.
8. Fowler ME. 1978. Peracute mortality in captive giraffe. J Am Vet Med Assoc. 173:1088–93.
9. Fowler ME. BWJ. 1986. Giraffidae (Giraffe and okapi). Zoo and Wild Animal Medicine. W.B. Saunders Co., Philadelphia.
10. Greenaway C, OP. 1996. A forborne outbreak causing a cholinergic syndrome. J Emerg Med. 14:339–44.
11. Junge RE. 1993. Peracute mortality syndrome of giraffes. Zoo and Wild Animal Medicine: Current Therapy. 3. Pp. 547–549. W.B. Saunders Company, Philadelphia, PA.
12. Kearney C, Ball, Ray. 2001. Body scoring system for captive Giraffe (Giraffa camelopardalis). Proceedings of the American Association of Zoo Veterinarians. Orlando, FL. 2001.
13. Lechowski R, J Pisarski, J Goslawski, M Lenarcik. 1991. Exocrine pancreatic insufficiency-like syndrome in giraffe. J Wildl Dis. 27:728–30.
14. Miller M, B Coville, N Abou-Madi, J Olsen. 1999. Comparison of in vitro tests for evaluation of passive transfer of immunoglobulins in giraffe (Giraffa camelopardalis). J Zoo Wild Med. 30:85–93.