Abstract
Pinnipeds play an important role in coastal regions around the world; they are high metabolism predator species with high consumption of food, associated to up-welling zones with high productivity.5 The study of adult females would reflect local environmental conditions because they remain most of the year near the reproductive areas, with long longevity. The raising of offspring can extend up to 1 yr or more, and thus a good body condition of the female would reflect in general, a good health condition of the pups.4 The health study of pups offers a practical alternative to indirectly evaluate the condition of the mothers and the general condition of the colony.11
The populations of the two species of pinnipeds found in the Galapagos islands, (Galapagos fur seal, Arctocephalus galapagoensis, and Galapagos sea lion, Zalophus wollebaeki) are relatively small, approximately 20,000–30,000 individuals and highly vulnerable to climatic changes, such as El Niño Southern Oscillation events.9,12,16,17 This had caused high mortality in pups in 1997 with a 99% of mortality.1 These pinnipeds, like other species of the archipelago, have a high risk of being affected by the introduction of exotic species (dogs, cats, and rats), and other problems, such as the oil spill that occurred on San Cristobal Island in January 2001, or a distemper outbreak in dogs reported in 2001 (Sandie Salazar, personal communication). In addition, the Galapagos sea lion is one of the main ecotourism highlights of the islands, due to the accessibility of the colonies. This increases their contact with international tourists, local residents and the interaction with solid waste, contaminated water and exotic species.
Long-term monitoring of health parameters of these populations would help to document changes in the prevalence of infectious agents and exposure to toxics. These data, together with general population dynamics and ecology, would provide essential information that enables the interpretation of the state of health of both of these pinniped populations and the ecosystem, with an interdisciplinary integration of knowledge.15
Since 2002, a field study has been carried out on pups during two reproductive seasons. Eleven and three rookeries have been monitored for the Galapagos sea lion and fur seal, respectively. Morphometric measurements, body weight, hair, blood, samples for microbiologic cultures from rectum and external lesions, and parasites were obtained under general isoflurane anesthesia.13,14
Serum samples are being tested for selected infectious pathogens known to occur in marine mammals. Testing for Brucella spp. included serology (ELISA), PCR and bacteriologic culture from blood, was negative for all cases. Serology was performed for leptospirosis using the microagglutination test and positive titers were found in Galapagos sea lions to pathogenic serovars autumnalis, bataviae, canicola, celledoni, copenhageni, grippotyphosa, hardjo-pratjino, pomona, and wolffi. Overall, seroprevalence was 71%. Copenhageni was the most represented serovar (55%). Highest antibody titers (1:200) were detected against serovars canicola and hardjo-pratjino, which were the only serovars found in Galapagos fur seals.
Leptospirosis is a zoonotic disease which occurs worldwide and is identified in humans, domestic livestock, terrestrial and marine mammals. The same pathogenic Leptospira serovar can infect humans and marine mammals.6,7 Populations of sea lions have experienced outbreaks of leptospirosis that cause significant mortality.8 Because the Galapagos Islands are a tourist destination for water-related activities, such as scuba diving and snorkeling, and as a destination for international scientists, there is a potential risk for susceptible individuals to come into contact with Leptospira. An infected host may eliminate up to 105 leptospires/ml urine during the first weeks of infection, and it has been reported that sea lions eliminate leptospires in the urine for up to 154 days after infection.2 Maintenance of leptospirosis in sea lions could be enhanced by crowding in rookeries and contact with urine.2
In addition, there is some concern with regards to animal welfare as perceived by tourists. For example, in the Galapagos sea lion colonies there is a high prevalence of purulent conjunctivitis mostly in pups that is frequently noted by the visitors. We identified this problem to be related with an ocular fluke, a digenetic trematode, in the genus Philophthalmus. The members of this genus are normally found in the eyes of birds. In some colonies, the prevalence of the parasite is as high as 92% of the pups examined. The presence of the eye parasites correlated with leukocytosis with a left shift. It is important to identify the potential carriers of this parasite in the Galapagos Islands, not only for the sea lions, but also as a public health hazard for tourists practicing water-related activities, because this parasite has been reported to infect humans.10
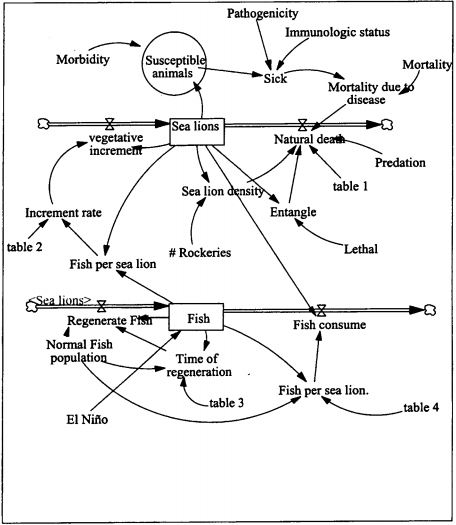
In order to better interpret the health status of both populations of pinnipeds, the results of this health survey are being compared to those obtained from a similar work done with California sea lions (Zalophus californianus) from the Gulf of Baja California, Mexico.4,7,11,13 An analysis of the information is being performed using a simulation model to assess the effect of diseases and other environmental factors like El Niño year on the population (Fig. 1). This will enable us to predict potential problems and identify missing information that could have a major impact in the population, and guide us in future research. The dynamics of infectious diseases and their ecological impacts in marine ecosystems are poorly understood. They can affect species of ecological value, and may cause mass mortalities or negatively affect the abundance and reproduction rates of host populations.2 However, these diseases can also be present in populations not in decline, depending on how they contribute to population dynamics. The development of an integrated regional monitoring program for a sentinel vertebrate species as an indicator of marine ecosystem health is making health and ecological professionals understand the interconnections of species and the complexity of ecological health problems.3,15
Simulation model for Galapagos sea lion (Zalophus wollebaeki)
Acknowledgments
The authors would like to acknowledge the team at The Charles Darwin Research Station, Galapagos Islands (Ecuador), and The Marine Mammal Center, Santa Barbara (California), and Adriana Flores for their support.
Literature Cited
1. A Special report From the Charles Darwin Research Station 2002, 1997 El Niño in Galapagos. www.darwinfoundation.org/articles/wc00039902.html. (VIN editor: URL was not accessible as of 3/2/2021).
2. Acevedo-Whitehouse K., H. de la Cueva, M. Frances, D. Gulland, D. Aureoles-Gamboa, F. Arellano-Carbajal, and F. Suarez-Guemes. 2003. Evidence of Leptospira interrogans infection in California sea lion pups from the gulf of California. J. Wildl. Dis. 39(1):145–151
3. Aguirre A.A., T.M O'Hara, T.R. Spraker, and D.A. Jessup. Monitoring the health and conservation of marine mammals, sea turtles and their ecosystems. In: Aguirre A.A., R.S. Ostfeld, G.M. Tabor, C. House. and M.C. Pearl. (eds). Conservation Medicine Ecological Health and Practice. 2002. Oxford University Press. 79–94.
4. Aurioles, G.D., I. G. Castro, F. R. Garcia, S. F. Luque, C.R. Godinez, D. Brousset, J. H. Montano, A. Paras, S. Montano, y F. R. Perez-Gil. 2000. Estado de salud de las poblaciones de lobo marino (Zalophus californianus) en el Golfo de California. Mem. del Primer Cong. de Responsables de Proyectos de Invest. en Ciencias Naturales, CONACYT. Veracruz Ver. Oct. 8–11
5. Costa P. D. The relationship between reproductive and foraging energetics and the evolution of the Pinnipedia., In: Boyd I. L. (Ed). 1993. Marine Mammals: Advances in Behavioral and Population Biology. The Zoological Society of London. Clarendon Press, Oxford, No. 66, 293–314.
6. Garcelon, D.K., R.K. Wayne, and B.J. Gonzales. 1992. A serologic survey of the island fox (Urocyon littoralis) on the Channel Islands, California. J. Wildl. Dis. 28:223–229.
7. Godinez, C.R., B. Zelaya de Romillo, D. Aurioloes-Gamboa, A. Verdugo-Rodriguez, E.A. Rodriquez-Reyes, and
8. A. De la Pena-Moctezuma. 1999. Antibodies against Leptospira interrogans in California sea lion pups from Gulf of California. J. Wildl. Dis. 35:108–111.
9. Gulland, F., M. Koski, L.J. Lowenstine, A. Colagross, L. Morgan, and T. Spraker.1996. Leptospirosis in California sea lions (Zalophus caifornianus) stranded along the central California coast. J. Wildl. Dis. 33:450–458.
10. Heath, R., R. DeLong, V. Jameson, D. Bradley, and T. Spraker. 1997. Isoflurane anesthesia in free ranging sea lion pups. J. Wild. Dis. 33:206–209.
11. Lang Y. Y Weiss, H. Garzozi, D. Gold, and J. Lengy. 1993. A first instance of human philophthalmosis in Israel. J. Helminothol. 67(2):107–111.
12. Luque F. S., y D. Aurioles. 2001. Sex differences in body size and body condition of California sea lion (Zalophus californianus) pups from the Gulf of California. Marine Mammal Science. 17(1):147–160.
13. Limberger, D., F. Trillmich, H. Biebach, and R. Stevenson. 1986. Temperature regulation and rnicrohabitat choice by free-ranging Galapagos fur seal pups (Arctocephalus galapagoensis). Oecologi. 69:53–59.
14. Paras, A., M.A. Benitez, D. M. Brousset, D. Aurioles, S. Luque, and C. Godinez. 1998. Anesthesia of California sea lions (Zalophus californianus) in eleven reproductive rookeries of the Gulf of California, Mexico. Proc. Am. Assoc. Zoo Vet. Pp. 425–430.
15. Paras, A., D. M. Brousset, S. Salazar and D. Aurioles. 2002. Field anaesthesia of two species ofpinnipeds (Arctocephalus galapagoensis and Zalophus wollebaeki) found in the Galapagos Islands. Proc. Am. Assoc. Zoo Vet. Pp. 431–433.
16. TaborG.M. Defining conservation medicine. In: Aguirre A.A., R.S. Ostfeld, G.M. Tabor, C. House. and M.C. Pearl. (eds). Conservation Medicine Ecological Health and Practice. 2002. Oxford University Press. Pp. 8–25.
17. Thowsend, C. H. 1934. The fur seal of the Galapagos Islands, Arctocephalus galapagoensis Heller. New York Zoological Society. Zoologica. 2:43–51.
18. Trillmich, F. 1979. Noticias de Galapagos. Estacion Cientifica Charles Darwin. 29:8–14.