Hypersensitivity to Captive Diets as a Possible Underlying Etiology for Clinical Signs and Pathology in Captive Black Rhinoceros (Diceros bicornis)
Abstract
Black rhinoceros in captivity have been plagued by a host of clinical entities. These include superficial necrolytic dermatitis (SND), hemosiderosis, hemolytic and non-hemolytic anemia, and recently the idiopathic hemorrhagic vasculopathy syndrome (IHVS) has been described in a group of black rhinoceros.3,4 Secondary infections are also noteworthy and include salmonellosisa, leptospirosis, tuberculosis, and aspergillosis.4 Information collected over the last several years have led to a theory that would potentially unite the pathologies and clinical conditions seen in captive black rhinoceros. A nutritional basis has long been suspected and the focus has tended to be on specific nutrients like iron or fatty acids.6,7 Another possibility is that captive diets may be an antigenic source and initiate a cascade of events that may lead to the pathologies and clinical conditions encountered in captive black rhinoceros.
Figure 1 summarizes the proposed pathophysiology with dietary hypersensitivity as the inciting cause for health issues seen in captive black rhinoceros. Differences exist in the level of inflammation between captive and wild black rhinoceros as evidenced with ferritin levels. The differences between wild rhinoceros and captive rhinoceros in regards to ferritin are well documented.6 While ferritin is used as a marker for tissue storage of iron, it is an acute phase inflammatory protein as well. Ferritin levels increase over time spent in captivity. This has been assumed to be to constant iron loading but a persistent inflammatory process could result in the same ferritin changes. A diet trial at Busch Gardens Tampa Bay (BGT) was undertaken in three adult male black rhinoceros (Diceros bicornis michaeli) (Studbook numbers: 518, 12-year-old; 0786, 5-year-old; 0864, 4-year-old) in which a commercial browser pellet was substituted with a low starch, high physical effective fiber diet designed for giraffes. Basic hematology, serum chemistries, serum ferritin, and immune profiles were collected. Serum ferritin levels were checked (Kansas State Veterinary Diagnostic Laboratory, Manhattan, KS, USA) at the beginning and ending of the trial and are listed in Table 2. The iron content of the new diet averages around 400 ppm but varies slightly between lots. The browser pellets contained was 370 ppm iron. Ferritin does spike with any inflammatory process, including immobilizations. On one occasion, rhinoceros 518 was immobilized for electroejaculation. Serum ferritin on that procedure was 5466 ng/ml. One week later it returned to “baseline” of 2443 ng/ml. Lymphocyte proliferation8 was evaluated at Mote Marine Laboratory, (Sarasota, FL, USA) at the beginning and end of the trial.8 Concanavalin A (Con A) and phytohemagglutinin (PHA) were used as mitogens to stimulate lymphocyte proliferation. Immune response was slightly less at the end of the 5-month time period. The difference may not be statistically significant but the clinical significance may be real given other inflammatory mediators had been reduced. Antiphospholipid antibodies (APhL) have not been evaluated as of this writing. A reduction in serum ferritin in spite of a higher iron diet suggests something other than iron intake is taking place here and a change in inflammation is suspected. Antiphospholipid antibodies (APhL) have been examined in captive and wild black rhinoceross, as well.1 Wild black rhinoceros have lower levels of these antibodies compared to captive ones. A rising level can be seen when young captive rhinoceros are weaned onto solid foods.1 This rise in APhL parallels that seen in ferritin. APhL are commonly seen in inflammatory processes in people. It is believed that in black rhinoceros they are reflective of an increased inflammatory stimulus in captivity. Given the various conditions wild rhinoceros are often in regards to parasites and wounds, a reasonable deduction would be the diet in captivity could be inciting the inflammation.
| Figure 1 | 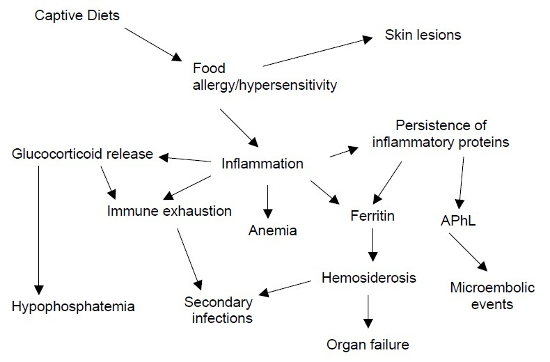
Proposed pathophysiology involving dietary hypersensitivity and gastrointestinal inflammation for the clinical and pathologic conditions in captive black rhinoceros. |
|
| |
Table 1. Food item hypersensitivity profiles on wild and captive black rhinoceros. (N=Negative ≤150, BL=Borderline 151–174, BL-P=Borderline-Positive 175–199, P=Positive 200–400, HP=Highly Positive >400). Dates from wild rhinoceros are approximates
|
|
518
|
0786
|
0786
|
0786
|
0786
|
Zim1
|
Zim2
|
Zim3
|
Zim4
|
Zim5
|
|
Sample date
|
07/12/01
|
12/21/00
|
10/22/04
|
02/28/05
|
12/14/05
|
04/01/01
|
04/01/01
|
04/01/01
|
04/01/01
|
04/01/01
|
|
Barley
|
129
|
100
|
237
|
256
|
261
|
100
|
134
|
100
|
100
|
111
|
|
Soy beans
|
140
|
164
|
149
|
176
|
196
|
129
|
100
|
138
|
100
|
135
|
|
Corn
|
159
|
138
|
237
|
214
|
239
|
149
|
243
|
165
|
165
|
177
|
|
Wheat
|
163
|
100
|
253
|
276
|
357
|
127
|
165
|
100
|
127
|
105
|
|
Brown rice
|
146
|
101
|
124
|
165
|
219
|
100
|
100
|
118
|
100
|
100
|
|
Potato
|
102
|
132
|
102
|
100
|
100
|
100
|
100
|
100
|
100
|
125
|
|
Beets
|
112
|
105
|
228
|
157
|
155
|
100
|
108
|
100
|
194
|
134
|
|
Carrot
|
117
|
100
|
149
|
142
|
166
|
100
|
100
|
100
|
100
|
100
|
|
Sweet potato
|
121
|
100
|
100
|
105
|
191
|
100
|
148
|
100
|
102
|
100
|
|
Yeast
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
101
|
100
|
100
|
|
Milk/cow’s
|
100
|
100
|
100
|
100
|
100
|
100
|
126
|
100
|
100
|
100
|
Table 2. Beginning and ending serum ferritin (ng/ml) from diet trial on three adult male captive black rhinoceros
|
|
Jan-05
|
Aug-05
|
|
518
|
4405
|
2490
|
|
786
|
10733
|
2860
|
|
864
|
12671
|
6052
|
Support for a dietary source of gastrointestinal inflammation also comes from recent field work. Eleven black rhinoceros were recently translocated from Hluhuwe-Imfolozi Wildlife Park in South Africa. At capture all rhinoceros had fecals collected for various projects. Fecal hemacult were analyzed using a commercial kit (Hemoccult®, Beckman Coulter, Inc., Fullerton, CA, USA) animal side for the presence of fecal occult blood. All eleven samples were negative. Fecal hemoccult tests are always positive in all species of rhinoceros at BGT. This test is not considered reliable in horses as the hindgut can readily degrade large amounts of hemoglobin, hence masking gastric bleeding.5 While negative results can possibly be false negatives, a false positive seems very unlikely and suggest some bleeding in the gastrointestinal tract. Tannins or other porporyin containing substances that could potentially interfere with this assay seem much more likely to occur in wild rhinoceros consuming natural browse material. A recent epidemiologic project looking at the health issues in captive black rhinoceros listed diarrhea as the most common problem seen.2 Food allergen testing has also been conducted at a commercial veterinary food allergen testing facility (Bio-Medical Services, Austin, TX, USA, www.bmslab.com) (VIN editor: Original link not accessible 1-26-2021) on captive black rhinoceros at BGT and five wild black rhinoceros from Zimbabwe. There is a fair amount of variability in the profiles between food items in the captive rhinoceros but corn and wheat are consistently reacting as antigens on the assay. A young captive born rhinoceros (61115) showeds an increase in the level of reactivity to several items over time with a large increase occurring after weaning. Most interesting is the large differences between the wild rhinoceros (Zim 1–5) and the captive rhinoceros. Persistence of these two inflammatory proteins, ferritin and APhL, may lead to problems directly. Antiphospholipid can cause microthromi and mimic problems seen in captive black rhinoceros.1 The problems with hemosiderosis are well documented in black rhinoceros.6
Acknowledgments
We would like to thank the Cathy Walsh at Mote Marine, Drs. Dave Cooper, Markus Hofmeyer, and Peter Buss in South Africa; Mary Port and Heather Henry at the Veterinary Hospital at BGT; and Jason Green, Kristin Forker, and Derek Weatherford and the entire rhinoceros crew at BGT.
Reprinted with permission of the Comparative Nutrition Society (CNS). 2006. Proceedings of the Sixth Biennial Conference, Keystone, Colorado. Information in the CNS abstracts is not peer reviewed and cannot be considered endorsed by the society.
Literature Cited
1. Ball RL, Morrow M, Hofmeyr PM, Buss P. Comparison of anti-phospholipid antibodies between wild and captive black rhinoceros (Bicornis bicornis): Implications for health and repatriation. In: Proceedings of the Institute for Zoo and Wildlife Research. 2005:72–74.
2. Dennis P, Saville WL, Blumer ES, et al. Preliminary review of an epidemiological survey of black rhinoceros in captivity in the United States. In: Proceedings of the American Association of Zoo Veterinarians Annual Meeting. 2003:247.
3. Kock N, Foggin C, Kock M, Kock R. Hemosiderosis in the black rhinoceros (Diceros bicornis): comparison of free-ranging and recently captured with translocated and captive animals. J Zoo Wildl Med. 1992;23:230–234.
4. Miller RE. Rhinoceridae. In: Fowler M, Miller RE, eds. Zoo and Wildlife Medicine, 5th ed. 2003:563–566.
5. Murray M. The gastrointestinal system. In: Robinson NE, ed. Current Therapy in Equine Medicine. Philadelphia, PA: W.B. Saunders Co.; 1997:169.
6. Paglia D, Dennis P. Role of chronic iron overload in multiple disorders of captive black rhinoceros (Diceros bicornis). In: Proceedings of the American Association of Zoo Veterinarians Annual Meeting. 1999:163–171.
7. Suedmeyer WK, Dierenfeld ES. Clinical experience with fatty acid supplementation in a group of black rhinoceros (Diceros bicornis) In: Proceedings of the American Association of Zoo Veterinarians Annual Meeting. 1998:113–115.
8. Vance CK, Kennedy-Stoskopf S, Obringer AR, Roth TL. Comparative studies of mitogen- and antigen-induced lymphocyte proliferation in four captive rhinoceros species. J Zoo Wildl Med. 2004;34(4):435–446.